Scientists in Singapore were inspired by dragonflies and cicadas according to a March 28, 2018 news item on Nanowerk (Note: A link has been removed),
Studies have shown that the wings of dragonflies and cicadas prevent bacterial growth due to their natural structure. The surfaces of their wings are covered in nanopillars making them look like a bed of nails. When bacteria come into contact with these surfaces, their cell membranes get ripped apart immediately and they are killed. This inspired researchers from the Institute of Bioengineering and Nanotechnology (IBN) of A*STAR to invent an anti-bacterial nano coating for disinfecting frequently touched surfaces such as door handles, tables and lift buttons.
This technology will prove particularly useful in creating bacteria-free surfaces in places like hospitals and clinics, where sterilization is important to help control the spread of infections. Their new research was recently published in the journal Small (“ZnO Nanopillar Coated Surfaces with Substrate-Dependent Superbactericidal Property”)
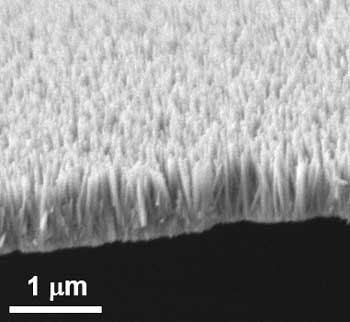
Image 1: Zinc oxide nanopillars that looked like a bed of nails can kill a broad range of germs when used as a coating on frequently-touched surfaces. Courtesy: A*STAR
A March 28, 2018 Agency for Science Technology and Research (A*STAR) press release, which originated the news item, describes the work further,
80% of common infections are spread by hands, according to the B.C. [province of Canada] Centre for Disease Control1. Disinfecting commonly touched surfaces helps to reduce the spread of harmful germs by our hands, but would require manual and repeated disinfection because germs grow rapidly. Current disinfectants may also contain chemicals like triclosan which are not recognized as safe and effective 2, and may lead to bacterial resistance and environmental contamination if used extensively.
“There is an urgent need for a better way to disinfect surfaces without causing bacterial resistance or harm to the environment. This will help us to prevent the transmission of infectious diseases from contact with surfaces,” said IBN Executive Director Professor Jackie Y. Ying.
To tackle this problem, a team of researchers led by IBN Group Leader Dr Yugen Zhang created a novel nano coating that can spontaneously kill bacteria upon contact. Inspired by studies on dragonflies and cicadas, the IBN scientists grew nanopilllars of zinc oxide, a compound known for its anti-bacterial and non-toxic properties. The zinc oxide nanopillars can kill a broad range of germs like E. coli and S. aureus that are commonly transmitted from surface contact.
Tests on ceramic, glass, titanium and zinc surfaces showed that the coating effectively killed up to 99.9% of germs found on the surfaces. As the bacteria are killed mechanically rather than chemically, the use of the nano coating would not contribute to environmental pollution. Also, the bacteria will not be able to develop resistance as they are completely destroyed when their cell walls are pierced by the nanopillars upon contact.
Further studies revealed that the nano coating demonstrated the best bacteria killing power when it is applied on zinc surfaces, compared with other surfaces. This is because the zinc oxide nanopillars catalyzed the release of superoxides (or reactive oxygen species), which could even kill nearby free floating bacteria that were not in direct contact with the surface. This super bacteria killing power from the combination of nanopillars and zinc broadens the scope of applications of the coating beyond hard surfaces.
Subsequently, the researchers studied the effect of placing a piece of zinc that had been coated with zinc oxide nanopillars into water containing E. coli. All the bacteria were killed, suggesting that this material could potentially be used for water purification.
Dr Zhang said, “Our nano coating is designed to disinfect surfaces in a novel yet practical way. This study demonstrated that our coating can effectively kill germs on different types of surfaces, and also in water. We were also able to achieve super bacteria killing power when the coating was used on zinc surfaces because of its dual mechanism of action. We hope to use this technology to create bacteria-free surfaces in a safe, inexpensive and effective manner, especially in places where germs tend to accumulate.”
IBN has recently received a grant from the National Research Foundation, Prime Minister’s Office, Singapore, under its Competitive Research Programme to further develop this coating technology in collaboration with Tan Tock Seng Hospital for commercial application over the next 5 years.
(I wasn’t expecting to see a reference to my home province [BC Centre for Disease Control].) Back to the usual, here’s a link to and a citation for the paper,
ZnO Nanopillar Coated Surfaces with Substrate‐Dependent Superbactericidal Property by Guangshun Yi, Yuan Yuan, Xiukai Li, Yugen Zhang. Small https://doi.org/10.1002/smll.201703159 First published: 22 February 2018
This paper is behind a paywall.
One final comment, this research reminds me of research into simulating shark skin because that too has bacteria-killing nanostructures. My latest about the sharkskin research is a Sept, 18, 2014 posting.