I think this form of ‘cannibalism’ could also be described as a form of ‘self-assembly’. That said, here is an August 31, 2018 news item on ScienceDaily announcing ‘cannibalistic’ materials,
Scientists at the [US] Department of Energy’s [DOE] Oak Ridge National Laboratory [ORNL] induced a two-dimensional material to cannibalize itself for atomic “building blocks” from which stable structures formed.
The findings, reported in Nature Communications, provide insights that may improve design of 2D materials for fast-charging energy-storage and electronic devices.
An August 31, 2018 DOE/Oak Ridge National Laboratory news release (also on EurekAlert), which originated the news item, provides more detail (Note: Links have been removed),
“Under our experimental conditions, titanium and carbon atoms can spontaneously form an atomically thin layer of 2D transition-metal carbide, which was never observed before,” said Xiahan Sang of ORNL.
He and ORNL’s Raymond Unocic led a team that performed in situ experiments using state-of-the-art scanning transmission electron microscopy (STEM), combined with theory-based simulations, to reveal the mechanism’s atomistic details.
“This study is about determining the atomic-level mechanisms and kinetics that are responsible for forming new structures of a 2D transition-metal carbide such that new synthesis methods can be realized for this class of materials,” Unocic added.
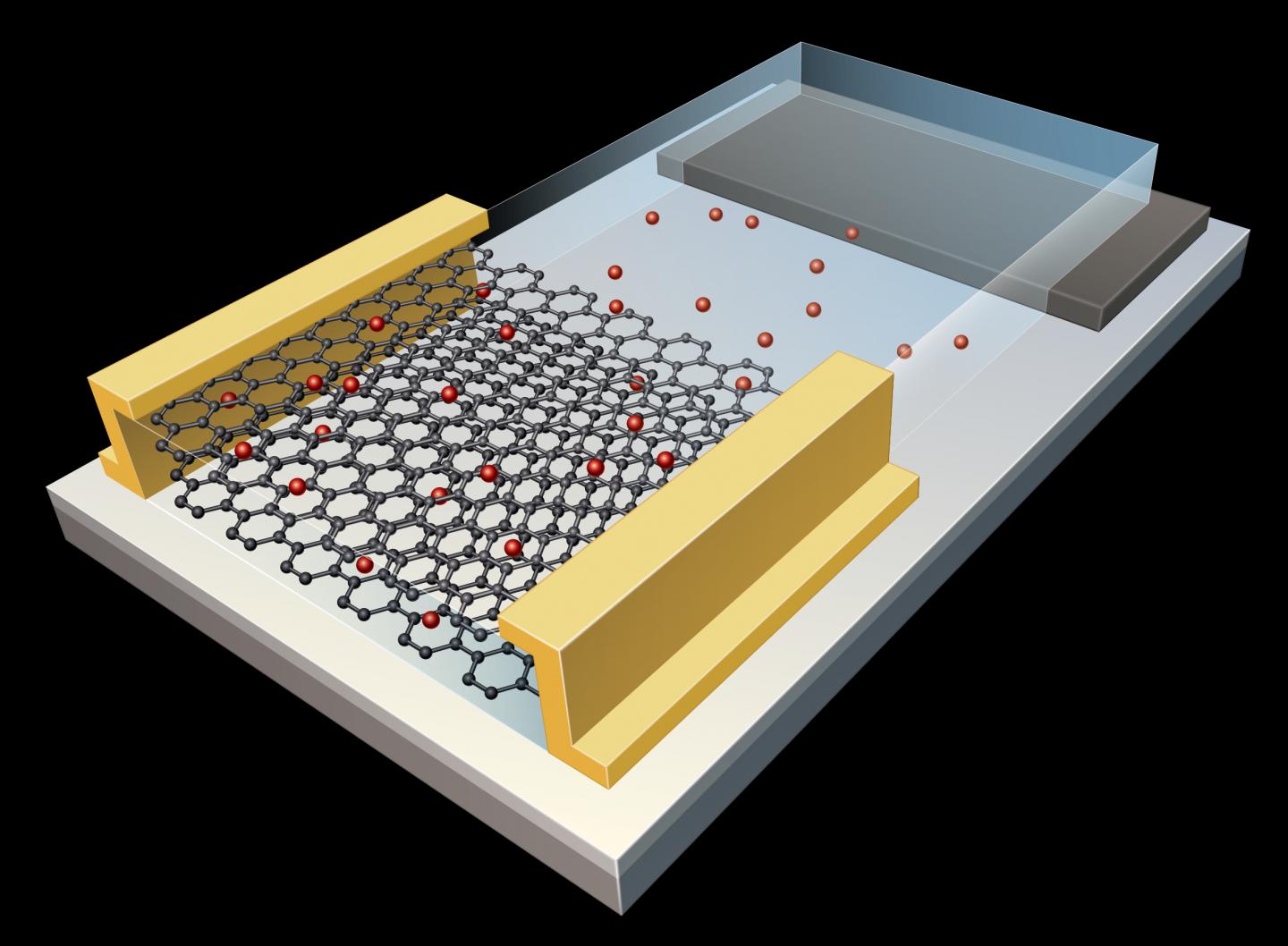
The starting material was a 2D ceramic called a MXene (pronounced “max een”). Unlike most ceramics, MXenes are good electrical conductors because they are made from alternating atomic layers of carbon or nitrogen sandwiched within transition metals like titanium.
The research was a project of the Fluid Interface Reactions, Structures and Transport (FIRST) Center, a DOE Energy Frontier Research Center that explores fluid–solid interface reactions that have consequences for energy transport in everyday applications. Scientists conducted experiments to synthesize and characterize advanced materials and performed theory and simulation work to explain observed structural and functional properties of the materials. New knowledge from FIRST projects provides guideposts for future studies.
The high-quality material used in these experiments was synthesized by Drexel University scientists, in the form of five-ply single-crystal monolayer flakes of MXene. The flakes were taken from a parent crystal called “MAX,” which contains a transition metal denoted by “M”; an element such as aluminum or silicon, denoted by “A”; and either a carbon or nitrogen atom, denoted by “X.” The researchers used an acidic solution to etch out the monoatomic aluminum layers, exfoliate the material and delaminate it into individual monolayers of a titanium carbide MXene (Ti3C2).
The ORNL scientists suspended a large MXene flake on a heating chip with holes drilled in it so no support material, or substrate, interfered with the flake. Under vacuum, the suspended flake was exposed to heat and irradiated with an electron beam to clean the MXene surface and fully expose the layer of titanium atoms.
MXenes are typically inert because their surfaces are covered with protective functional groups—oxygen, hydrogen and fluorine atoms that remain after acid exfoliation. After protective groups are removed, the remaining material activates. Atomic-scale defects—“vacancies” created when titanium atoms are removed during etching—are exposed on the outer ply of the monolayer. “These atomic vacancies are good initiation sites,” Sang said. “It’s favorable for titanium and carbon atoms to move from defective sites to the surface.” In an area with a defect, a pore may form when atoms migrate.
“Once those functional groups are gone, now you’re left with a bare titanium layer (and underneath, alternating carbon, titanium, carbon, titanium) that’s free to reconstruct and form new structures on top of existing structures,” Sang said.
High-resolution STEM imaging proved that atoms moved from one part of the material to another to build structures. Because the material feeds on itself, the growth mechanism is cannibalistic.
“The growth mechanism is completely supported by density functional theory and reactive molecular dynamics simulations, thus opening up future possibilities to use these theory tools to determine the experimental parameters required for synthesizing specific defect structures,” said Adri van Duin of Penn State [Pennsylvania State University].
Most of the time, only one additional layer [of carbon and titanium] grew on a surface. The material changed as atoms built new layers. Ti3C2 turned into Ti4C3, for example.
“These materials are efficient at ionic transport, which lends itself well to battery and supercapacitor applications,” Unocic said. “How does ionic transport change when we add more layers to nanometer-thin MXene sheets?” This question may spur future studies.
“Because MXenes containing molybdenum, niobium, vanadium, tantalum, hafnium, chromium and other metals are available, there are opportunities to make a variety of new structures containing more than three or four metal atoms in cross-section (the current limit for MXenes produced from MAX phases),” Yury Gogotsi of Drexel University added. “Those materials may show different useful properties and create an array of 2D building blocks for advancing technology.”
At ORNL’s Center for Nanophase Materials Sciences (CNMS), Yu Xie, Weiwei Sun and Paul Kent performed first-principles theory calculations to explain why these materials grew layer by layer instead of forming alternate structures, such as squares. Xufan Li and Kai Xiao helped understand the growth mechanism, which minimizes surface energy to stabilize atomic configurations. Penn State scientists conducted large-scale dynamical reactive force field simulations showing how atoms rearranged on surfaces, confirming defect structures and their evolution as observed in experiments.
The researchers hope the new knowledge will help others grow advanced materials and generate useful nanoscale structures.
Here’s a link to and a citation for the paper,
In situ atomistic insight into the growth mechanisms of single layer 2D transition metal carbides by Xiahan Sang, Yu Xie, Dundar E. Yilmaz, Roghayyeh Lotfi, Mohamed Alhabeb, Alireza Ostadhossein, Babak Anasori, Weiwei Sun, Xufan Li, Kai Xiao, Paul R. C. Kent, Adri C. T. van Duin, Yury Gogotsi, & Raymond R. Unocic. Nature Communicationsvolume 9, Article number: 2266 (2018) DOI: https://doi.org/10.1038/s41467-018-04610-0 Published 11 June 2018
This paper is open access.