A May 18, 2022 news item on phys.org highlights the problem with using hydrogen as an energy source,
The effects of global warming are becoming more serious, and there is a strong demand for technological advances to reduce carbon dioxide emissions. Hydrogen is an ideal clean energy which produces water when burned. To promote the use of hydrogen energy, it is essential to develop safe, energy-saving technologies for hydrogen production and storage. Currently, hydrogen is made from natural gas, so it is not appropriate for decarbonization. Using a lot of energy to separate hydrogen would not make it qualify as clean energy.
Polymer separation membranes have the great advantage of enlarging the separation membrane and increasing the separation coefficient. However, the speed of permeation through the membrane is extremely low, and high pressure must be applied to increase the permeation speed. Therefore, a large amount of energy is required for separation using a polymer separation membrane. The goal is to create a new kind of separation membrane technology that can achieve separation speeds that are 50 times faster than that of conventional separation membranes.
A May 18, 2022 Shinshu University (Japan) press release on EurekAlert, which originated the news item, describes a proposed solution to the hydrogen problem,
The graphene-wrapped molecular-sieving membrane prepared in this study has a separation factor of 245 and a permeation coefficient of 5.8 x 106 barrers, which is more than 100 times better than that of conventional polymer separation membranes. If the size of the separation membrane is increased in the future, it is very probable that an energy-saving separation process will be established for the separation of important gases such as carbon dioxide and oxygen as well as hydrogen.
As seen in the transmission electron microscope image in Figure 1 [not shown], graphene is wrapped around the MFI-type zeolite crystal, being hydrophobic. The wrapping uses the principles of colloidal science to keep graphene and zeolite crystal planes close to each other due to reduction of the repulsive interaction. About 5 layers of graphene enclose zeolite crystals in this figure. Around the red arrow, there is a narrow interface space where only hydrogen can permeate. Graphene is also present on hydrophobic zeolite, so the structure of the zeolite crystal cannot be seen with this. Since a strong attractive force acts between graphene, the zeolite crystals wrapped with graphene are in close contact with each other by a simple compression treatment and does not let any gas through.
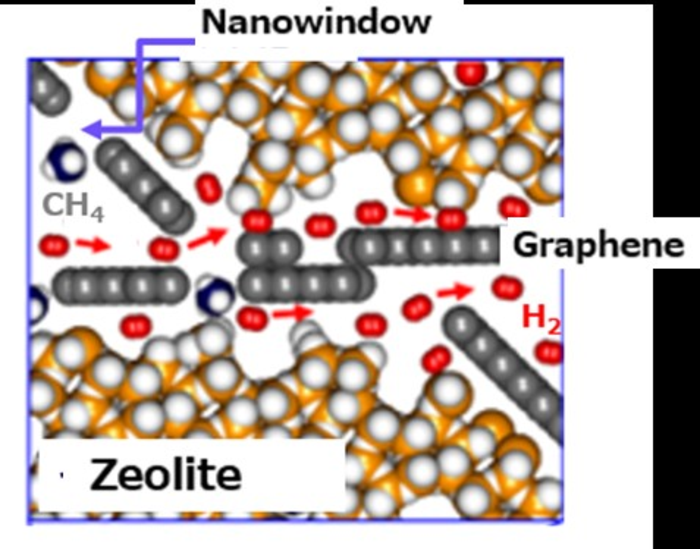
Figure 2 [not shown] shows a model in which zeolite crystals wrapped with graphene are in contact with each other. The surface of the zeolite crystal has grooves derived from the structure, and there is an interfacial channel between zeolite and graphene through which hydrogen molecules can selectively permeate. The model in which the black circles are connected is graphene, and there are nano-windows represented by blanks in some places. Any gas can freely permeate the nanowindows, but the very narrow channels between graphene and zeolite crystal faces allow hydrogen to permeate preferentially. This structure allows efficient separation of hydrogen and methane. On the other hand, the movement of hydrogen is rapid because there are many voids between the graphene-wrapped zeolite particles. For this reason, ultra-high-speed permeation is possible while maintaining the high separation factor of 200 or more.
Figure 3 [not shown] compares the hydrogen separation factor and gas permeation coefficient for methane with the previously reported separation membranes, which is called Robeson plot. Therefore, this separation membrane separates hydrogen at a speed of about 100 times while maintaining a higher separation coefficient than conventional separation membranes. The farther in the direction of the arrow, the better the performance. This newly developed separation membrane has paved the way for energy-saving separation technologies for the first time.
In addition, this separation principle is different from the conventional dissolution mechanism with polymers and the separation mechanism with pore size in zeolite separation membranes, and it depends on the separation target by selecting the surface structure of zeolite or another crystal. High-speed separation for any target gas is possible in principle. For this reason, if the industrial manufacturing method of this separation membrane and the separation membrane becomes scalable, the chemical industry, combustion industry, and other industries can be significantly improved energy consumption, leading to a significant reduction in carbon dioxide emissions. Currently, the group is conducting research toward the establishment of basic technology for rapidly producing a large amount of enriched oxygen from air. The development of enriched oxygen manufacturing technologies will revolutionize the steel and chemical industry and even medicine.
The figures referenced in the press release are best seen in the context of the paper. I can show you part of Figure 1,
For the rest of Figure 1 and more figures, here’s a link to and a citation for the paper,
Ultrapermeable 2D-channeled graphene-wrapped zeolite molecular sieving membranes for hydrogen separation by Radovan Kukobat, Motomu Sakai, Hideki Tanaka, Hayato Otsuka, Fernando Vallejos-Burgos, Christian Lastoskie, Masahiko Matsukata, Yukichi Sasaki, Kaname Yoshida, Takuya Hayashi and Katsumi Kaneko. Science Advances 18 May 2022 Vol 8, Issue 20 DOI: 10.1126/sciadv.abl3521
This paper is open access.