I hadn’t heard of counterfeit seeds before but this March 22, 2023 news item on phys.org describes the problem and announces an approach to prevention,
Average crop yields in Africa are consistently far below expected, and one significant reason is the prevalence of counterfeit seeds whose germination rates are far lower than those of the genuine ones. The World Bank estimates that as much as half of all seeds sold in some African countries are fake, which could help to account for crop production that is far below potential.
There have been many attempts to prevent this counterfeiting through tracking labels, but none have proved effective; among other issues, such labels have been vulnerable to hacking because of the deterministic nature of their encoding systems. But now, a team of MIT [Massachusetts Institute of Technology] researchers has come up with a kind of tiny, biodegradable tag that can be applied directly to the seeds themselves, and that provides a unique randomly created code that cannot be duplicated.
…
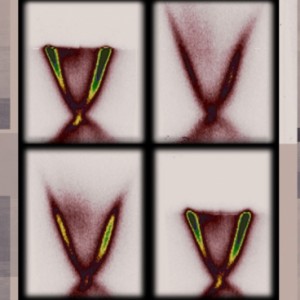
I found this picture helped me to understand how ‘tracking’ these seeds could be useful,

A March 22, 2023 Massachusetts Institute of Technology (MIT) news release by David Chandler (also on EurekAlert), which originated the news item, describes the work in more detail (Note: I’m glad to see David Chandler is getting bylines again even if it’s on the EurekAlert version only),
The new system, which uses minuscule dots of silk-based material, each containing a unique combination of different chemical signatures, is described today in the journal Science Advances in a paper by MIT’s dean of engineering Anantha Chandrakasan, professor of civil and environmental engineering Benedetto Marelli, postdoc Hui Sun, and graduate student Saurav Maji.
The problem of counterfeiting is an enormous one globally, the researchers point out, affecting everything from drugs to luxury goods, and many different systems have been developed to try to combat this. But there has been less attention to the problem in the area of agriculture, even though the consequences can be severe. In sub-Saharan Africa, for example, the World Bank estimates that counterfeit seeds are a significant factor in crop yields that average less than one-fifth of the potential for maize, and less than one-third for rice.
Marelli explains that a key to the new system is creating a randomly-produced physical object whose exact composition is virtually impossible to duplicate. The labels they create “leverage randomness and uncertainty in the process of application, to generate unique signature features that can be read, and that cannot be replicated,” he says.
What they’re dealing with, Sun adds, “is the very old job of trying, basically, not to get your stuff stolen. And you can try as much as you can, but eventually somebody is always smart enough to figure out how to do it, so nothing is really unbreakable. But the idea is, it’s almost impossible, if not impossible, to replicate it, or it takes so much effort that it’s not worth it anymore.”
The idea of an “unclonable” code was originally developed as a way of protecting the authenticity of computer chips, explains Chandrakasan, who is the Vannevar Bush Professor of Electrical Engineering and Computer Science. “In integrated circuits, individual transistors have slightly different properties coined device variations,” he explains, “and you could then use that variability and combine that variability with higher-level circuits to create a unique ID for the device. And once you have that, then you can use that unique ID as a part of a security protocol. Something like transistor variability is hard to replicate from device to device, so that’s what gives it its uniqueness, versus storing a particular fixed ID.” The concept is based on what are known as physically unclonable functions, or PUFs.
The team decided to try to apply that PUF principle to the problem of fake seeds, and the use of silk proteins was a natural choice because the material is not only harmless to the environment but also classified by the Food and Drug Administration in the “generally recognized as safe” category, so it requires no special approval for use on food products.
“You could coat it on top of seeds,” Maji says, “and if you synthesize silk in a certain way, it will also have natural random variations. So that’s the idea, that every seed or every bag could have a unique signature.”
Developing effective secure system solutions have long been one of Chandrakasan’s specialties, while Marelli has spent many years developing systems for applying silk coatings to a variety of fruits, vegetables, and seeds, so their collaboration was a natural for developing such a silk-based coding system towards enhanced security.
“The challenge was what type of form factor to give to silk,” Sun says, “so that it can be fabricated very easily.” They developed a simple drop-casting approach that produces tags that are less than one-tenth of an inch in diameter. The second challenge was to develop “a way where we can read the uniqueness, in also a very high throughput and easy way.”
For the unique silk-based codes, Marelli says, “eventually we found a way to add a color to these microparticles [emphasis mine] so that they assemble in random structures.” The resulting unique patterns can be read out not only by a spectrograph or a portable microscope, but even by an ordinary cellphone camera with a macro lens. This image can be processed locally to generate the PUF code and then sent to the cloud and compared with a secure database to ensure the authenticity of the product. “It’s random so that people cannot easily replicate it,” says Sun. “People cannot predict it without measuring it.”
And the number of possible permutations that could result from the way they mix four basic types of colored silk nanoparticles [emphasis mine] is astronomical. “We were able to show that with a minimal amount of silk, we were able to generate 128 random bits of security,” Maji says. “So this gives rise to 2 to the power 128 possible combinations, which is extremely difficult to crack given the computational capabilities of the state-of-the-art computing systems.”
Marelli says that “for us, it’s a good test bed in order to think out-of-the-box, and how we can have a path that somehow is more democratic.” In this case, that means “something that you can literally read with your phone, and you can fabricate by simply drop casting a solution, without using any advanced manufacturing technique, without going in a clean room.”
Some additional work will be needed to make this a practical commercial product, Chandrakasan says. “There will have to be a development for at-scale reading” via smartphones. “So. that’s clearly a future opportunity.” But the principle now shows a clear path to the day when “a farmer could at least, maybe not every seed, but could maybe take some random seeds in a particular batch and verify them,” he says.
I’ve looked at the paper (very quickly) and haven’t spotted any mention of silk nanoparticles. It’s all silk microparticles.
Here’s a link to and a citation for the paper,
Integrating biopolymer design with physical unclonable functions for anticounterfeiting and product traceability in agriculture by Hui Sun, Saurav Maji, Anantha P. Chandrakasan, and Benedetto Marelli. Science Advances 22 Mar 2023 Vol 9, Issue 12 DOI: 10.1126/sciadv.adf1978
This paper is open access.