Contrast agents used for magnetic resonance imaging, x-ray imaging, ultrasounds, and other imaging technologies are not always kind to the humans ingesting them. So, scientists at the University at Buffalo (also known as the State University of New York at Buffalo) have developed a veggie juice that does the job according to a July 11, 2016 news item on Nanowerk (Note: A link has been removed),
The pigment that gives spinach and other plants their verdant color may improve doctors’ ability to examine the human gastrointestinal tract.
That’s according to a study, published in the journal Advanced Materials (“Surfactant-Stripped Frozen Pheophytin Micelles for Multimodal Gut Imaging”), which describes how chlorophyll-based nanoparticles suspended in liquid are an effective imaging agent for the gut.
The University of Buffalo has provided an illustration of the work,
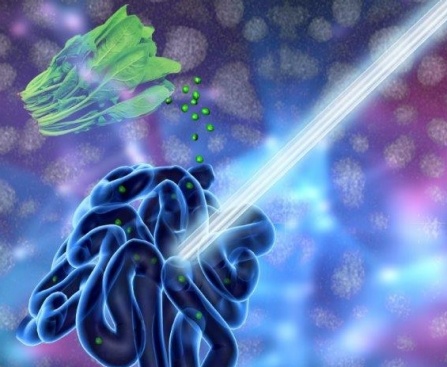
A new UB-led study suggests that chlorophyll-based nanoparticles are an effective imaging agent for the gut. The medical imaging drink, developed to diagnose and treat gastrointestinal illnesses, is made of concentrated chlorophyll, the pigment that makes spinach green. Photo illustration credit: University at Buffalo.
A July 11, 2016 University at Buffalo (UB) news release (also on EurekAlert) by Cory Nealon, which originated the news item, expands on the theme,
“Our work suggests that this spinach-like, nanoparticle juice can help doctors get a better look at what’s happening inside the stomach, intestines and other areas of the GI tract,” says Jonathan Lovell, PhD, assistant professor in the Department of Biomedical Engineering, a joint program between UB’s School of Engineering and Applied Sciences and the Jacobs School of Medicine and Biomedical Sciences at UB, and the study’s corresponding author.
To examine the gastrointestinal tract, doctors typically use X-rays, magnetic resonance imaging or ultrasounds, but these techniques are limited with respect to safety, accessibility and lack of adequate contrast, respectively.
Doctors also perform endoscopies, in which a tiny camera attached to a thin tube is inserted into the patient’s body. While effective, this procedure is challenging to perform in the small intestine, and it can cause infections, tears and pose other risks.
The new study, which builds upon Lovell’s previous medical imaging research, is a collaboration between researchers at UB and the University of Wisconsin-Madison. It focuses on Chlorophyll a, a pigment found in spinach and other green vegetables that is essential to photosynthesis.
In the laboratory, researchers removed magnesium from Chlorophyll a, a process which alters the pigment’s chemical structure to form another edible compound called pheophytin. Pheophytin plays an important role in photosynthesis, acting as a gatekeeper that allows electrons from sunlight to enter plants.
Next, they dissolved pheophytin in a solution of soapy substances known as surfactants. The researchers were then able to remove nearly all of the surfactants, leaving nearly pure pheophytin nanoparticles.
The drink, when tested in mice, provided imaging of the gut in three modes: photoacoustic imaging, fluorescence imaging and positron emission tomography (PET). (For PET, the researchers added to the drink Copper-64, an isotope of the metal that, in small amounts, is harmless to the human body.)
Additional studies are needed, but the drink has commercial potential because it:
· Works in different imaging techniques.
· Moves stably through the gut.
· And is naturally consumed in the human diet already.
In lab tests, mice excreted 100 percent of the drink in photoacoustic and fluorescence imaging, and nearly 93 percent after the PET test.
“The veggie juice allows for techniques that are not commonly used today by doctors for imaging the gut like photoacoustic, PET, and fluorescence,” Lovell says. “And part of the appeal is the safety of the juice.”
Here’s a link to and a citation for the paper,
Surfactant-Stripped Frozen Pheophytin Micelles for Multimodal Gut Imaging by Yumiao Zhang, Depeng Wang, Shreya Goel, Boyang Sun, Upendra Chitgupi, Jumin Geng, Haiyan Sun, Todd E. Barnhart, Weibo Cai, Jun Xia, and Jonathan F. Lovell. Advanced Materials DOI: 10.1002/adma.201602373 Version of Record online: 11 JUL 2016
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
This paper is behind a paywall.
![Schematic of the two-organ system [downloaded from http://www.rsc.org/chemistryworld/2014/07/nanoparticle-liver-gastrointestinal-tract-microfluidic-chip]](http://www.frogheart.ca/wp-content/uploads/2014/08/TwoOrganSystem.jpg)