“As good as gold” was a behavioural goal when I was a child. It turns out, the same can be said of gold in electronic devices according to the headline for a March 26, 2020 news item on Nanowerk (Note: Links have been removed),
As electronics shrink to nanoscale, will they still be good as gold?
Deep inside computer chips, tiny wires made of gold and other conductive metals carry the electricity used to process data.
But as these interconnected circuits shrink to nanoscale, engineers worry that pressure, such as that caused by thermal expansion when current flows through these wires, might cause gold to behave more like a liquid than a solid, making nanoelectronics unreliable. That, in turn, could force chip designers to hunt for new materials to make these critical wires.
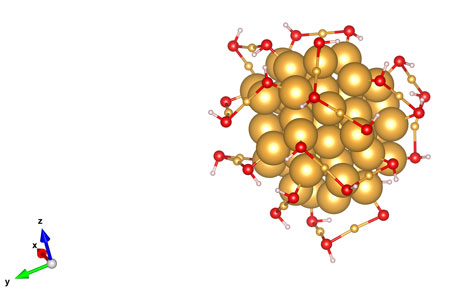
But according to a new paper in Physical Review Letters (“Nucleation of Dislocations in 3.9 nm Nanocrystals at High Pressure”), chip designers can rest easy. “Gold still behaves like a solid at these small scales,” says Stanford mechanical engineer Wendy Gu, who led a team that figured out how to pressurize gold particles just 4 nanometers in length — the smallest particles ever measured — to assess whether current flows might cause the metal’s atomic structure to collapse.
I have seen the issue about gold as a metal or liquid before but I can’t find it here (search engines, sigh). However, I found this somewhat related story from almost five years ago. In my April 14, 2015 posting (Gold atoms: sometimes they’re a metal and sometimes they’re a molecule), there was news that the number of gold atoms present means the difference between being a metal and being a molecule .This could have implications as circuit elements (which include some gold in their fabrication) shrink down past a certain point.
A March 24, 2020 Stanford University news release (also on Eurekalert but published on March 25, 2020) by Andrew Myers, which originated the news item, provides details about research designed to investigate a similar question, i.e, can we used gold as we shrink the scale?*,
To conduct the experiment, Gu’s team first had to devise a way put tiny gold particles under extreme pressure, while simultaneously measuring how much that pressure damaged gold’s atomic structure.
To solve the first problem, they turned to the field of high-pressure physics to borrow a device known as a diamond anvil cell. As the name implies, both hammer and anvil are diamonds that are used to compress the gold. As Gu explained, a nanoparticle of gold is built like a skyscraper with atoms forming a crystalline lattice of neat rows and columns. She knew that pressure from the anvil would dislodge some atoms from the crystal and create tiny defects in the gold.
The next challenge was to detect these defects in nanoscale gold. The scientists shined X-rays through the diamond onto the gold. Defects in the crystal caused the X-rays to reflect at different angles than they would on uncompressed gold. By measuring variations in the angles at which the X-rays bounced off the particles before and after pressure was applied, the team was able to tell whether the particles retained the deformations or reverted to their original state when pressure was lifted.
In practical terms, her findings mean that chipmakers can know with certainty that they’ll be able to design stable nanodevices using gold — a material they have known and trusted for decades — for years to come.
“For the foreseeable future, gold’s luster will not fade,” Gu says.
*The 2015 research measured the gold nanoclusters by the number of atoms within the cluster with the changes occurring at some where between 102 atoms and 144 atoms. This 2020 work measures the amount of gold by nanometers as in 3.9 nm gold nanocrystals . So, how many gold atoms in a nanometer? Cathy Murphy provides the answer and the way to calculate it for yourself in a July 26, 2016 posting on the Sustainable Nano blog ( a blog by the Center for Sustainable Nanotechnology),
Two years ago, I wrote a blog post called Two Ways to Make Nanoparticles, describing the difference between top-down and bottom-up methods for making nanoparticles. In the post I commented, “we can estimate, knowing how gold atoms pack into crystals, that there are about 2000 gold atoms in one 4 nm diameter gold nanoparticle.” Recently, a Sustainable Nano reader wrote in to ask about how this calculation is done. It’s a great question!
…
So, a 3.9 nm gold nanocrystal contains approximately 2000 gold atoms. (If you have time, do read Murphy’s description of how to determine the number of gold atoms in a gold nanoparticle.) So, this research does not answer the question posed by the 2015 research.
It may take years before researchers can devise tests for gold nanoclusters consisting of 102 atoms as opposed to nanoparticles consisting of 2000 atoms. In the meantime, here’s a link to and a citation for the latest on how gold reacts as we shrink the size of our electronics,
Nucleation of Dislocations in 3.9 nm Nanocrystals at High Pressure by Abhinav Parakh, Sangryun Lee, K. Anika Harkins, Mehrdad T. Kiani, David Doan, Martin Kunz, Andrew Doran, Lindsey A. Hanson, Seunghwa Ryu, and X. Wendy Gu. Phys. Rev. Lett. 124, 106104 DOI:https://doi.org/10.1103/PhysRevLett.124.106104 Published 13 March 2020 © 2020 American Physical Society
This paper is behind a paywall.