I believe that swab they stick up your nose to test for COVDI-19 is 10 inches long so it seems to me that discomfort or unpleasant are not the words that best describe the testing experience .
Hopefully, no one will have to find inadequate vocabulary for this new COVID-19 testing assuming that future trials are successful and they are able to put the technology into production. From an August 19, 2020 news item on Nanowerk,
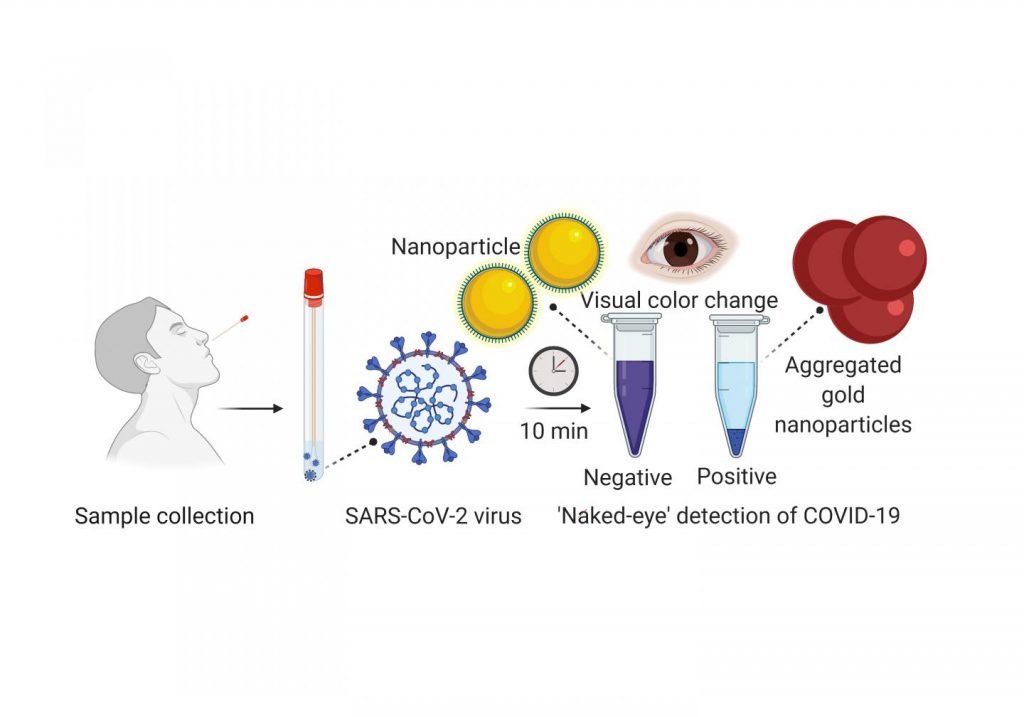
Few people who have undergone nasopharyngeal swabs for coronavirus testing would describe it as a pleasant experience. The procedure involves sticking a long swab up the nose to collect a sample from the back of the nose and throat, which is then analyzed for SARS-CoV-2 RNA [ribonucleic acid] by the reverse-transcription polymerase chain reaction (RT-PCR).
Now, researchers reporting in [American Chemical Society] ACS Nano (“Multiplexed Nanomaterial-Based Sensor Array for Detection of COVID-19 in Exhaled Breath”) have developed a prototype device that non-invasively detected COVID-19 in the exhaled breath of infected patients.
…
An August 19, 2020 ACS news release (also received via email and on EurekAlert), which originated the news item, provides more technical details,
In addition to being uncomfortable, the current gold standard for COVID-19 testing requires RT-PCR, a time-consuming laboratory procedure. Because of backlogs, obtaining a result can take several days. To reduce transmission and mortality rates, healthcare systems need quick, inexpensive and easy-to-use tests. Hossam Haick, Hu Liu, Yueyin Pan and colleagues wanted to develop a nanomaterial-based sensor that could detect COVID-19 in exhaled breath, similar to a breathalyzer test for alcohol intoxication. Previous studies have shown that viruses and the cells they infect emit volatile organic compounds (VOCs) that can be exhaled in the breath.
The researchers made an array of gold nanoparticles linked to molecules that are sensitive to various VOCs. When VOCs interact with the molecules on a nanoparticle, the electrical resistance changes. The researchers trained the sensor to detect COVID-19 by using machine learning to compare the pattern of electrical resistance signals obtained from the breath of 49 confirmed COVID-19 patients with those from 58 healthy controls and 33 non-COVID lung infection patients in Wuhan, China. Each study participant blew into the device for 2-3 seconds from a distance of 1¬-2 cm. Once machine learning identified a potential COVID-19 signature, the team tested the accuracy of the device on a subset of participants. In the test set, the device showed 76% accuracy in distinguishing COVID-19 cases from controls and 95% accuracy in discriminating COVID-19 cases from lung infections. The sensor could also distinguish, with 88% accuracy, between sick and recovered COVID-19 patients. Although the test needs to be validated in more patients, it could be useful for screening large populations to determine which individuals need further testing, the researchers say.
The authors acknowledge funding from the Technion-Israel Institute of Technology.
…
Here’s a link to and a citation for the paper,
Multiplexed Nanomaterial-Based Sensor Array for Detection of COVID-19 in Exhaled Breath by Benjie Shan, Yoav Y Broza, Wenjuan Li, Yong Wang, Sihan Wu, Zhengzheng Liu, Jiong Wang, Shuyu Gui, Lin Wang, Zhihong Zhang, Wei Liu, Shoubing Zhou, Wei Jin, Qianyu Zhang, Dandan Hu, Lin Lin, Qiujun Zhang, Wenyu Li, Jinquan Wang, Hu Liu, Yueyin Pan, and Hossam Haick. ACS Nano 2020, XXXX, XXX, XXX-XXX DOI: https://doi.org/10.1021/acsnano.0c05657 Publication Date:August 18, 2020 Copyright © 2020 American Chemical Society
This paper is behind a paywall.