For the 5% or less of the world who need this explanation, the reference to a football later in this post is, in fact, a reference to a soccer ball. Moving on to a Nov. 5, 2014 news item on Nanowerk (Note: A link has been removed),
Using DESY’s ultrabright X-ray source PETRA III, researchers have observed in real-time how football-shaped carbon molecules arrange themselves into ultra-smooth layers. Together with theoretical simulations, the investigation reveals the fundamentals of this growth process for the first time in detail, as the team around Sebastian Bommel (DESY and Humboldt Universität zu Berlin) and Nicola Kleppmann (Technische Universität Berlin) reports in the scientific journal Nature Communications (“Unravelling the multilayer growth of the fullerene C60 in real-time”).
This knowledge will eventually enable scientists to tailor nanostructures from these carbon molecules for certain applications, which play an increasing role in the promising field of plastic electronics. The team consisted of scientists from Humboldt-Universität zu Berlin, Technische Universität Berlin, Universität Tübingen and DESY.
Here’s an image of the self-assembling materials,
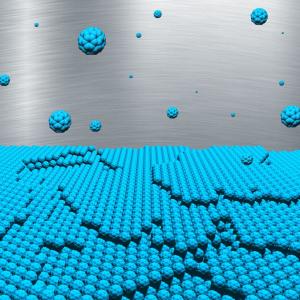
Caption: This is an artist’s impression of the multilayer growth of buckyballs.
Credit: Nicola Kleppmann/TU Berlin
A Nov. 5, 2014 DESY (Deutsches Elektronen-Synchrotron) press release (also on EurekAlert), describes the work further,
The scientists studied so called buckyballs. Buckyballs are spherical molecules, which consist of 60 carbon atoms (C60). Because they are reminiscent of American architect Richard Buckminster Fuller’s geodesic domes, they were christened buckminsterfullerenes or “buckyballs” for short. With their structure of alternating pentagons and hexagons, they also resemble tiny molecular footballs. [emphasis mine]
Using DESY’s X-ray source PETRA III, the researchers observed how buckyballs settle on a substrate from a molecular vapour. In fact, one layer after another, the carbon molecules grow predominantly in islands only one molecule high and barely form tower-like structures..“The first layer is 99% complete before 1% of the second layer is formed,” explains DESY researcher Bommel, who is completing his doctorate in Prof. Stefan Kowarik’s group at the Humboldt Universität zu Berlin. This is how extremely smooth layers form.
“To really observe the growth process in real-time, we needed to measure the surfaces on a molecular level faster than a single layer grows, which takes place in about a minute,” says co-author Dr. Stephan Roth, head of the P03 measuring station, where the experiments were carried out. “X-ray investigations are well suited, as they can trace the growth process in detail.”
“In order to understand the evolution of the surface morphology at the molecular level, we carried out extensive simulations in a non-equilibrium system. These describe the entire growth process of C60 molecules into a lattice structure,” explains Kleppmann, PhD student in Prof. Sabine Klapp’s group at the Institute of Theoretical Physics, Technische Universität Berlin. “Our results provide fundamental insights into the molecular growth processes of a system that forms an important link between the world of atoms and that of colloids.”
Through the combination of experimental observations and theoretical simulations, the scientists determined for the first time three major energy parameters simultaneously for such a system: the binding energy between the football molecules, the so-called “diffusion barrier,” which a molecule must overcome if it wants to move on the surface, and the Ehrlich-Schwoebel barrier, which a molecule must overcome if it lands on an island and wants to hop down from that island.
“With these values, we now really understand for the first time how such nanostructures come into existence,” stresses Bommel. “Using this knowledge, it is conceivable that these structures can selectively be grown in the future: How must I change my temperature and deposition rate parameters so that an island of a particular size will grow. This could, for example, be interesting for organic solar cells, which contain C60.” The researchers intend to explore the growth of other molecular systems in the future using the same methods.
Here’s a link to and a citation for the paper,
Unravelling the multilayer growth of the fullerene C60 in real time by S. Bommel, N. Kleppmann, C. Weber, H. Spranger, P. Schäfer, J. Novak, S.V. Roth, F. Schreiber, S.H.L. Klapp, & S. Kowarik. Nature Communications 5, Article number: 5388 doi:10.1038/ncomms6388 Published 05 November 2014
This article is open access.
I was not able to find any videos of these buckyballs assembling in real-time. Presumably, there are technical issues with recording the process, financial issues, or some combination thereof. Still, I can’t help but feel teased (tongue in cheek) by these scientists who give me an artist’s concept instead. Hopefully, budgets and/or technology will allow the rest of us to view this process at some time in the future.