Sometimes the urge for wordplay overwhelms me as it did this morning (June 12, 2014) when I saw llamas mentioned in a news item. For anyone unfamiliar with how Canadian English (and I can safely include American English here but am not sure about any other Englishes) is spoken, we leave out consonants in some phrases. For example, ‘let me’ becomes ‘lemme’, which when you’re playing with ‘llama,’ becomes ‘llam’me. As for the verb ‘lend’, I used it for its alliterative quality and used more accurate verb ‘extracted’ later in the headline.
Getting on to the antibodies and the camels and llamas, here’s more from a June 12, 2014 news item on Nanowerk (Note: A link has been removed),
The use of nanoparticles in cancer research is considered as a promising approach in detecting and fighting tumour cells. The method has, however, often failed because the human immune system recognizes the particles as foreign objects and rejects them before they can fulfil their function. Researchers at the Helmholtz-Zentrum Dresden-Rossendorf (HZDR) and at University College Dublin [UCD[ in Ireland have, along with other partners, developed nanoparticles that not only bypass the body’s defence system, but also find their way to the diseased cells (“Diagnostic nanoparticle targeting of the EGF-receptor in complex biological conditions using single-domain antibodies”). This procedure uses fragments from a particular type of antibody that only occurs in camels and llamas. The small particles were even successful under conditions which are very similar to the situation within potential patients’ bodies.
A June 12, 2014 HZDR press release, which originated the news item, supplies a quote from one of the researchers where he explains the problems he and his colleagues were attempting to address,
Describing the current state of research, Dr. Kristof Zarschler of the Helmholtz Virtual Institute NanoTracking at the HZDR explains, “At the moment we must overcome three challenges. First, we need to produce the smallest possible nanoparticles. We then need to modify their surface in a way that the proteins in the human bodies do not envelop them, which would thus render them ineffective. In order to ensure, that the particles do their job, we must also somehow program them to find the diseased cells.” Therefore, the Dresden [HZDR is in Dresden] and Dublin researchers combined expertise to develop nanoparticles made of silicon dioxide with fragments of camel antibodies.
The press release and Zarschler go on to explain the advantages of camel and llama antibodies,
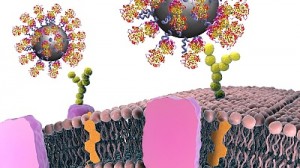
In contrast to conventional antibodies, which consist of two light and two heavy protein chains, those taken from camels and llamas are less complex and are made up of only two heavy chains. “Due to this simplified structure, they are easier to produce than normal antibodies,” explains Zarschler. “We also only need one particular fragment – the portion of the molecule that binds to certain cancer cells – which makes the production of much smaller nanoparticles possible.” By modifying the surface of the nanoparticle, it also gets more difficult for the immune system to recognize the foreign material, which allows the nanoparticles to actually reach their target.
The ultra-small particles should then detect the so-called epidermal growth factor receptor (EGFR) in the human body. In various types of tumours, this molecule is overexpressed and/or exists in a mutated form, which allows the cells to grow and multiply uncontrollably. The Dresden researchers could demonstrate in experiments that nanoparticles that have been combined with the camel antibody fragments can more firmly bind to the cancer cells. “The EGFR is a virtual lock to which our antibody fits like a key,” explains Zarschler.
Most exciting are the experiments the researchers performed with human blood (from the press release),
They even obtained the same results in experiments involving human blood serum – a biologically relevant environment the scientists point out: “This means that we carried out the tests under conditions that are very similar to the reality of the human body,” explains Dr. Holger Stephan, who leads the project. “The problem with many current studies is that artificial conditions are chosen where no disruptive factors exist. While this provides good results, it is ultimately useless because the nanoparticles fail finally in experiments conducted under more complex conditions. In our case, we could at least reduce this error source.”
There are no immediate plans for clinical trials according to the press release,
However, more time is required before the nanoparticles can be utilized in diagnosing human tumours. “The successful tests have brought us one step further,” explains Stephan. “The road, however, to its clinical use is long.” The next aim is to reduce the size of the nanoparticles, which are now approximately fifty nanometres in diameter, to less than ten nanometres. “That would be optimal,” according to Zarschler. “Then they would only remain in the human body for a short period – just long enough to detect the tumour.”
Here’s a link to and a citation for the paper,
Diagnostic nanoparticle targeting of the EGF-receptor in complex biological conditions using single-domain antibodies by K. Zarschler, K. Prapainop, E. Mahon, L. Rocks, M. Bramini, P. M. Kelly, H. Stephan, and K. A. Dawson. Nanoscale, 2014,6, 6046-6056 DOI: 10.1039/C4NR00595C
First published online 16 Apr 2014
This paper is in an open access journal.
The researchers have provided an illustration of the new antibody particles,