The French have announced some research into memristive devices that mimic both short-term and long-term neural plasticity according to a Dec. 6, 2016 news item on Nanowerk,
Leti researchers have demonstrated that memristive devices are excellent candidates to emulate synaptic plasticity, the capability of synapses to enhance or diminish their connectivity between neurons, which is widely believed to be the cellular basis for learning and memory.
The breakthrough was presented today [Dec. 6, 2016] at IEDM [International Electron Devices Meeting] 2016 in San Francisco in the paper, “Experimental Demonstration of Short and Long Term Synaptic Plasticity Using OxRAM Multi k-bit Arrays for Reliable Detection in Highly Noisy Input Data”.
Neural systems such as the human brain exhibit various types and time periods of plasticity, e.g. synaptic modifications can last anywhere from seconds to days or months. However, prior research in utilizing synaptic plasticity using memristive devices relied primarily on simplified rules for plasticity and learning.
The project team, which includes researchers from Leti’s sister institute at CEA Tech, List, along with INSERM and Clinatec, proposed an architecture that implements both short- and long-term plasticity (STP and LTP) using RRAM devices.
A Dec. 6, 2016 Laboratoire d’électronique des technologies de l’information (LETI) press release, which originated the news item, elaborates,
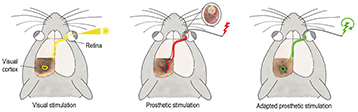
“While implementing a learning rule for permanent modifications – LTP, based on spike-timing-dependent plasticity – we also incorporated the possibility of short-term modifications with STP, based on the Tsodyks/Markram model,” said Elisa Vianello, Leti non-volatile memories and cognitive computing specialist/research engineer. “We showed the benefits of utilizing both kinds of plasticity with visual pattern extraction and decoding of neural signals. LTP allows our artificial neural networks to learn patterns, and STP makes the learning process very robust against environmental noise.”
Resistive random-access memory (RRAM) devices coupled with a spike-coding scheme are key to implementing unsupervised learning with minimal hardware footprint and low power consumption. Embedding neuromorphic learning into low-power devices could enable design of autonomous systems, such as a brain-machine interface that makes decisions based on real-time, on-line processing of in-vivo recorded biological signals. Biological data are intrinsically highly noisy and the proposed combined LTP and STP learning rule is a powerful technique to improve the detection/recognition rate. This approach may enable the design of autonomous implantable devices for rehabilitation purposes
Leti, which has worked on RRAM to develop hardware neuromorphic architectures since 2010, is the coordinator of the H2020 [Horizon 2020] European project NeuRAM3. That project is working on fabricating a chip with architecture that supports state-of-the-art machine-learning algorithms and spike-based learning mechanisms.
That’s it folks.