Gold, at the nanoscale, has different properties than it has at the macroscale and research at the University of Cambridge has found a new way to exploit gold’s unique properties at the nanoscale according to a May 13, 2019 news item item on ScienceDaily,
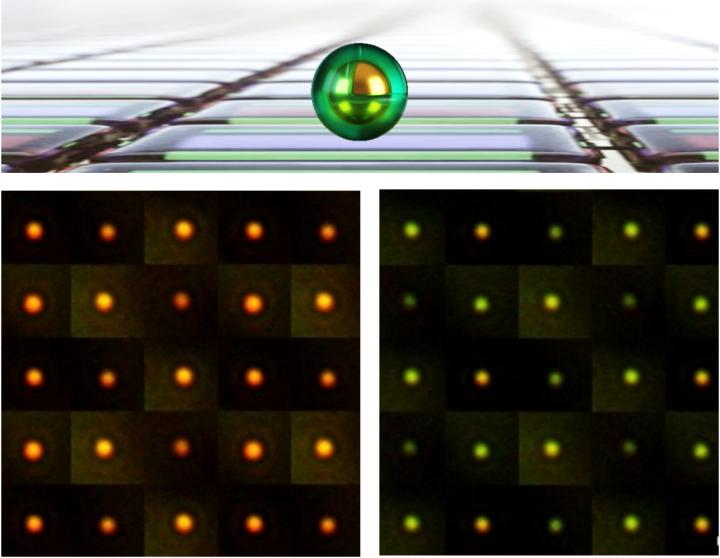
The smallest pixels yet created — a million times smaller than those in smartphones, made by trapping particles of light under tiny rocks of gold — could be used for new types of large-scale flexible displays, big enough to cover entire buildings.
The colour pixels, developed by a team of scientists led by the University of Cambridge, are compatible with roll-to-roll fabrication on flexible plastic films, dramatically reducing their production cost. The results are reported in the journal Science Advances [May 10, 2019].
…
A May 10,2019 University of Cambridge press release (also on EurekAlert), which originated the news item, delves further into the research,
It has been a long-held dream to mimic the colour-changing skin of octopus or squid, allowing people or objects to disappear into the natural background, but making large-area flexible display screens is still prohibitively expensive because they are constructed from highly precise multiple layers.
At the centre of the pixels developed by the Cambridge scientists is a tiny particle of gold a few billionths of a metre across. The grain sits on top of a reflective surface, trapping light in the gap in between. Surrounding each grain is a thin sticky coating which changes chemically when electrically switched, causing the pixel to change colour across the spectrum.
The team of scientists, from different disciplines including physics, chemistry and manufacturing, made the pixels by coating vats of golden grains with an active polymer called polyaniline and then spraying them onto flexible mirror-coated plastic, to dramatically drive down production cost.
The pixels are the smallest yet created, a million times smaller than typical smartphone pixels. They can be seen in bright sunlight and because they do not need constant power to keep their set colour, have an energy performance that makes large areas feasible and sustainable. “We started by washing them over aluminized food packets, but then found aerosol spraying is faster,” said co-lead author Hyeon-Ho Jeong from Cambridge’s Cavendish Laboratory.
“These are not the normal tools of nanotechnology, but this sort of radical approach is needed to make sustainable technologies feasible,” said Professor Jeremy J Baumberg of the NanoPhotonics Centre at Cambridge’s Cavendish Laboratory, who led the research. “The strange physics of light on the nanoscale allows it to be switched, even if less than a tenth of the film is coated with our active pixels. That’s because the apparent size of each pixel for light is many times larger than their physical area when using these resonant gold architectures.”
The pixels could enable a host of new application possibilities such as building-sized display screens, architecture which can switch off solar heat load, active camouflage clothing and coatings, as well as tiny indicators for coming internet-of-things devices.
The team are currently working at improving the colour range and are looking for partners to develop the technology further.The research is funded as part of a UK Engineering and Physical Sciences Research Council (EPSRC) investment in the Cambridge NanoPhotonics Centre, as well as the European Research Council (ERC) and the China Scholarship Council.
This image accompanies the press release,
Here’s a link to and a citation for the paper,
Scalable electrochromic nanopixels using plasmonics by Jialong Peng, Hyeon-Ho Jeong, Qianqi Lin, Sean Cormier, Hsin-Ling Liang, Michael F. L. De Volder, Silvia Vignolini, and Jeremy J. Baumberg. Science Advances Vol. 5, no. 5, eaaw2205 DOI: 10.1126/sciadv.aaw2205 Published: 01 May 2019
This paper appears to be open access.
![AGELESS BRILLIANCE: Although the pigment-derived leaf color of this decades-old specimen of the African perennial Pollia condensata has faded, the fruit still maintains its intense metallic-blue iridescence.COURTESY OF P.J. RUDALL [downloaded from http://www.the-scientist.com/?articles.view/articleNo/34200/title/Color-from-Structure/]](http://www.frogheart.ca/wp-content/uploads/2014/06/AgelessColour_-Pollia_condensata_Herbarium2.jpg)