This looks like interesting work and I think the integration of visual images and embedded video in the news release (on the university website) is particularly well done. I won’t be including all the graphical information here as my focus is the text.
A Sept. 10, 2020 City University of Hong Kong (CityU) press release (also on EurekAlert) announces a greener, more effective face mask,
Face masks have become an important tool in fighting against the COVID-19 pandemic. However, improper use or disposal of masks may lead to “secondary transmission”. A research team from City University of Hong Kong (CityU) has successfully produced graphene masks with an anti-bacterial efficiency of 80%, which can be enhanced to almost 100% with exposure to sunlight for around 10 minutes. Initial tests also showed very promising results in the deactivation of two species of coronaviruses. The graphene masks are easily produced at low cost, and can help to resolve the problems of sourcing raw materials and disposing of non-biodegradable masks.
The research is conducted by Dr Ye Ruquan, Assistant Professor from CityU’s Department of Chemistry, in collaboration with other researchers. The findings were published in the scientific journal ACS Nano, titled “Self-Reporting and Photothermally Enhanced Rapid Bacterial Killing on a Laser-Induced Graphene Mask“.
Commonly used surgical masks are not anti-bacterial. This may lead to the risk of secondary transmission of bacterial infection when people touch the contaminated surfaces of the used masks or discard them improperly. Moreover, the melt-blown fabrics used as a bacterial filter poses an impact on the environment as they are difficult to decompose. Therefore, scientists have been looking for alternative materials to make masks.
Converting other materials into graphene by laser
Dr Ye has been studying the use of laser-induced graphene [emphasis mine] in developing sustainable energy. When he was studying PhD degree at Rice University several years ago, the research team he participated in and led by his supervisor discovered an easy way to produce graphene. They found that direct writing on carbon-containing polyimide films (a polymeric plastic material with high thermal stability) using a commercial CO2 infrared laser system can generate 3D porous graphene. The laser changes the structure of the raw material and hence generates graphene. That’s why it is named laser-induced graphene.
Graphene is known for its anti-bacterial properties, so as early as last September, before the outbreak of COVID-19, producing outperforming masks with laser-induced graphene already came across Dr Ye’s mind. He then kick-started the study in collaboration with researchers from the Hong Kong University of Science and Technology (HKUST), Nankai University, and other organisations.
Excellent anti-bacterial efficiency
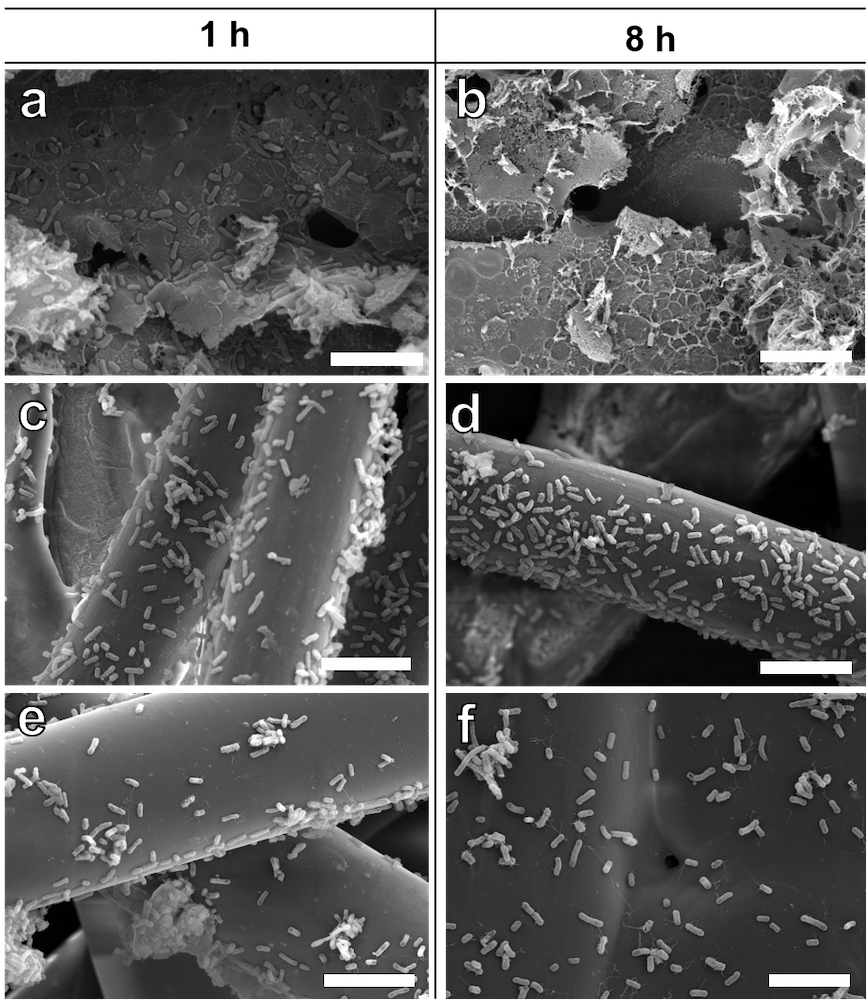
The research team tested their laser-induced graphene with E. coli, and it achieved high anti-bacterial efficiency of about 82%. In comparison, the anti-bacterial efficiency of activated carbon fibre and melt-blown fabrics, both commonly-used materials in masks, were only 2% and 9% respectively. Experiment results also showed that over 90% of the E. coli deposited on them remained alive even after 8 hours, while most of the E. coli deposited on the graphene surface were dead after 8 hours. Moreover, the laser-induced graphene showed a superior anti-bacterial capacity for aerosolised bacteria.
Dr Ye said that more research on the exact mechanism of graphene’s bacteria-killing property is needed. But he believed it might be related to the damage of bacterial cell membranes by graphene’s sharp edge. And the bacteria may be killed by dehydration induced by the hydrophobic (water-repelling) property of graphene.
Previous studies suggested that COVID-19 would lose its infectivity at high temperatures. So the team carried out experiments to test if the graphene’s photothermal effect (producing heat after absorbing light) can enhance the anti-bacterial effect. The results showed that the anti-bacterial efficiency of the graphene material could be improved to 99.998% within 10 minutes under sunlight, while activated carbon fibre and melt-blown fabrics only showed an efficiency of 67% and 85% respectively.
The team is currently working with laboratories in mainland China to test the graphene material with two species of human coronaviruses. Initial tests showed that it inactivated over 90% of the virus in five minutes and almost 100% in 10 minutes under sunlight. The team plans to conduct testings with the COVID-19 virus later.
Their next step is to further enhance the anti-virus efficiency and develop a reusable strategy for the mask. They hope to release it to the market shortly after designing an optimal structure for the mask and obtaining the certifications.
Dr Ye described the production of laser-induced graphene as a “green technique”. All carbon-containing materials, such as cellulose or paper, can be converted into graphene using this technique. And the conversion can be carried out under ambient conditions without using chemicals other than the raw materials, nor causing pollution. And the energy consumption is low.
“Laser-induced graphene masks are reusable. If biomaterials are used for producing graphene, it can help to resolve the problem of sourcing raw material for masks. And it can lessen the environmental impact caused by the non-biodegradable disposable masks,” he added.
Dr Ye pointed out that producing laser-induced graphene is easy. Within just one and a half minutes, an area of 100 cm² can be converted into graphene as the outer or inner layer of the mask. Depending on the raw materials for producing the graphene, the price of the laser-induced graphene mask is expected to be between that of surgical mask and N95 mask. He added that by adjusting laser power, the size of the pores of the graphene material can be modified so that the breathability would be similar to surgical masks.
A new way to check the condition of the mask
To facilitate users to check whether graphene masks are still in good condition after being used for a period of time, the team fabricated a hygroelectric generator. It is powered by electricity generated from the moisture in human breath. By measuring the change in the moisture-induced voltage when the user breathes through a graphene mask, it provides an indicator of the condition of the mask. Experiment results showed that the more the bacteria and atmospheric particles accumulated on the surface of the mask, the lower the voltage resulted. “The standard of how frequently a mask should be changed is better to be decided by the professionals. Yet, this method we used may serve as a reference,” suggested Dr Ye.
Laser-induced graphene (LIG), Rice University, and Dr. Ye were mentioned here in a May 9, 2018 titled: Do you want that coffee with some graphene on toast?
Back to the latest research, read the caption carefully,
Here’s a link to and a citation for the paper,
Self-Reporting and Photothermally Enhanced Rapid Bacterial Killing on a Laser-Induced Graphene Mask by Libei Huang, Siyu Xu, Zhaoyu Wang, Ke Xue, Jianjun Su, Yun Song, Sijie Chen, Chunlei Zhu, Ben Zhong Tang, and Ruquan Ye. ACS Nano 2020, 14, 9, 12045–12053 DOI: https://doi.org/10.1021/acsnano.0c05330 Publication Date:August 11, 2020 Copyright © 2020 American Chemical Society
This paper is behind a paywall.