Queensland University of Technology* (QUT; Australia) researchers are hopeful they can adapt supercapacitors in the form of a fine film tor use in electric vehicles making them more energy-efficient. From a Nov. 6, 2014 news item on ScienceDaily,
A car powered by its own body panels could soon be driving on our roads after a breakthrough in nanotechnology research by a QUT team.
Researchers have developed lightweight “supercapacitors” that can be combined with regular batteries to dramatically boost the power of an electric car.
The discovery was made by Postdoctoral Research Fellow Dr Jinzhang Liu, Professor Nunzio Motta and PhD researcher Marco Notarianni, from QUT’s Science and Engineering Faculty — Institute for Future Environments, and PhD researcher Francesca Mirri and Professor Matteo Pasquali, from Rice University in Houston, in the United States.
A Nov. 6, 2014 QUT news release, which originated the news item, describes supercapacitors, the research, and the need for this research in more detail,
The supercapacitors – a “sandwich” of electrolyte between two all-carbon electrodes – were made into a thin and extremely strong film with a high power density.
The film could be embedded in a car’s body panels, roof, doors, bonnet and floor – storing enough energy to turbocharge an electric car’s battery in just a few minutes.
…
“Vehicles need an extra energy spurt for acceleration, and this is where supercapacitors come in. They hold a limited amount of charge, but they are able to deliver it very quickly, making them the perfect complement to mass-storage batteries,” he said.
“Supercapacitors offer a high power output in a short time, meaning a faster acceleration rate of the car and a charging time of just a few minutes, compared to several hours for a standard electric car battery.”
Dr Liu said currently the “energy density” of a supercapacitor is lower than a standard lithium ion (Li-Ion) battery, but its “high power density”, or ability to release power in a short time, is “far beyond” a conventional battery.
“Supercapacitors are presently combined with standard Li-Ion batteries to power electric cars, with a substantial weight reduction and increase in performance,” he said.
“In the future, it is hoped the supercapacitor will be developed to store more energy than a Li-Ion battery while retaining the ability to release its energy up to 10 times faster – meaning the car could be entirely powered by the supercapacitors in its body panels.
“After one full charge this car should be able to run up to 500km – similar to a petrol-powered car and more than double the current limit of an electric car.”
Dr Liu said the technology would also potentially be used for rapid charges of other battery-powered devices.
“For example, by putting the film on the back of a smart phone to charge it extremely quickly,” he said.
The discovery may be a game-changer for the automotive industry, with significant impacts on financial, as well as environmental, factors.
“We are using cheap carbon materials to make supercapacitors and the price of industry scale production will be low,” Professor Motta said.
“The price of Li-Ion batteries cannot decrease a lot because the price of Lithium remains high. This technique does not rely on metals and other toxic materials either, so it is environmentally friendly if it needs to be disposed of.”
A Nov. 10, 2014 news item on Azonano describes the Rice University (Texas, US) contribution to this work,
Rice University scientist Matteo Pasquali and his team contributed to two new papers that suggest the nano-infused body of a car may someday power the car itself.
Rice supplied high-performance carbon nanotube films and input on the device design to scientists at the Queensland University of Technology in Australia for the creation of lightweight films containing supercapacitors that charge quickly and store energy. The inventors hope to use the films as part of composite car doors, fenders, roofs and other body panels to significantly boost the power of electric vehicles.
A Nov. 7, 2014 Rice University news release, which originated the news item, offers a few technical details about the film being proposed for use as a supercapacitor on car panels,
Researchers in the Queensland lab of scientist Nunzio Motta combined exfoliated graphene and entangled multiwalled carbon nanotubes combined with plastic, paper and a gelled electrolyte to produce the flexible, solid-state supercapacitors.
“Nunzio’s team is making important advances in the energy-storage area, and we were glad to see that our carbon nanotube film technology was able to provide breakthrough current collection capability to further improve their devices,” said Pasquali, a Rice professor of chemical and biomolecular engineering and chemistry. “This nice collaboration is definitely bottom-up, as one of Nunzio’s Ph.D. students, Marco Notarianni, spent a year in our lab during his Master of Science research period a few years ago.”
“We built on our earlier work on CNT films published in ACS Nano, where we developed a solution-based technique to produce carbon nanotube films for transparent electrodes in displays,” said Francesca Mirri, a graduate student in Pasquali’s research group and co-author of the papers. “Now we see that carbon nanotube films produced by the solution-processing method can be applied in several areas.”
As currently designed, the supercapacitors can be charged through regenerative braking and are intended to work alongside the lithium-ion batteries in electric vehicles, said co-author Notarianni, a Queensland graduate student.
“Vehicles need an extra energy spurt for acceleration, and this is where supercapacitors come in. They hold a limited amount of charge, but with their high power density, deliver it very quickly, making them the perfect complement to mass-storage batteries,” he said.
Because hundreds of film supercapacitors are used in the panel, the electric energy required to power the car’s battery can be stored in the car body. “Supercapacitors offer a high power output in a short time, meaning a faster acceleration rate of the car and a charging time of just a few minutes, compared with several hours for a standard electric car battery,” Notarianni said.
The researchers foresee such panels will eventually replace standard lithium-ion batteries. “In the future, it is hoped the supercapacitor will be developed to store more energy than an ionic battery while retaining the ability to release its energy up to 10 times faster – meaning the car would be powered by the supercapacitors in its body panels,” said Queensland postdoctoral researcher Jinzhang Liu.
Here’s an image of graphene infused with carbon nantoubes used in the supercapacitor film,
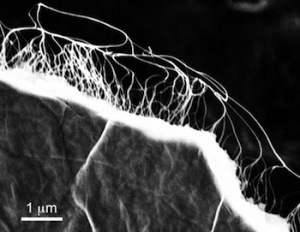
A scanning electron microscope image shows freestanding graphene film with carbon nanotubes attached. The material is part of a project to create lightweight films containing super capacitors that charge quickly and store energy. Courtesy of Nunzio Motta/Queensland University of Technology
Here are links to and citations for the two papers published by the researchers,
Graphene-based supercapacitor with carbon nanotube film as highly efficient current collector by Marco Notarianni, Jinzhang Liu, Francesca Mirri, Matteo Pasquali, and Nunzio Motta. Nanotechnology Volume 25 Number 43 doi:10.1088/0957-4484/25/43/435405
High performance all-carbon thin film supercapacitors by Jinzhang Liu, Francesca Mirri, Marco Notarianni, Matteo Pasquali, and Nunzio Motta. Journal of Power Sources Volume 274, 15 January 2015, Pages 823–830 DOI: 10.1016/j.jpowsour.2014.10.104
Both articles are behind paywalls.
One final note, Dexter Johnson provides some insight into issues with graphene-based supercapacitors and what makes this proposed application attractive in his Nov. 7, 2014 post on the Nanoclast blog (on the IEEE [Institute of Electrical and Electronics Engineers] website; Note: Links have been removed),
The hope has been that someone could make graphene electrodes for supercapacitors that would boost their energy density into the range of chemical-based batteries. The supercapacitors currently on the market have on average an energy density around 28 Wh/kg, whereas a Li-ion battery holds about 200Wh/kg. That’s a big gap to fill.
The research in the field thus far has indicated that graphene’s achievable surface area in real devices—the factor that determines how many ions a supercapacitor electrode can store, and therefore its energy density—is not any better than traditional activated carbon. In fact, it may not be much better than a used cigarette butt.
Though graphene may not help increase supercapacitors’ energy density, its usefulness in this application may lie in the fact that its natural high conductivity will allow superconductors to operate at higher frequencies than those that are currently on the market. Another likely benefit that graphene will yield comes from the fact that it can be structured and scaled down, unlike other supercapacitor materials.
I recommend reading Dexter’s commentary in its entirety.
*’University of Queensland’ corrected to “Queensland University of Technology’ on Nov. 10, 2014 at 1335 PST.
* ‘super-capacitor’ changed to ‘supercapacitor’ on April 29, 2015.
