English: Tattoo of Hand of Fatima,. Model: Casini. Date: 4 July 2017, 18:13:41. Source : Own work. Author: Stephencdickson.
For those who like their news in video format, there’s this Canadian Broadcasting Corporation (CBC) news item broadcast on Sep. 11, 2017 (after the commercials),
For those who like text and more detail, scientists at the European Synchrotron Radiation Facility (ESRF) have produced a study of the (at the nanoparticle scale) inks in tattoos. From a Sept. 12, 2017 news item on phys.org,
The elements that make up the ink in tattoos travel inside the body in micro and nanoparticle forms and reach the lymph nodes, according to a study published in Scientific Reports on 12 September [2017] by scientists from Germany and the ESRF, the European Synchrotron, Grenoble (France). It is the first time researchers have found analytical evidence of the transport of organic and inorganic pigments and toxic element impurities as well as in depth characterization of the pigments ex vivo in tattooed tissues. Two ESRF beamlines were crucial in this breakthrough.
A Sept. 12, 2017 ESRF press release (also on EurkeAlert), which originated the news item, explains further,
The reality is that little is known about the potential impurities in the colour mixture applied to the skin. Most tattoo inks contain organic pigments, but also include preservatives and contaminants like nickel, chromium, manganese or cobalt. Besides carbon black, the second most common ingredient used in tattoo inks is titanium dioxide (TiO2), a white pigment usually applied to create certain shades when mixed with colorants. Delayed healing, along with skin elevation and itching, are often associated with white tattoos, and by consequence with the use of TiO2. TiO2 is also commonly used in food additives, sun screens and paints. Scientists from the ESRF, the German Federal Institute for Risk Assessment, Ludwig-Maximilians University, and the Physikalisch-Technische Bundesanstalt have managed to get a very clear picture on the location of titanium dioxide once it gets in the tissue. This work was done on the ESRF beamlines ID21 and ID16B.
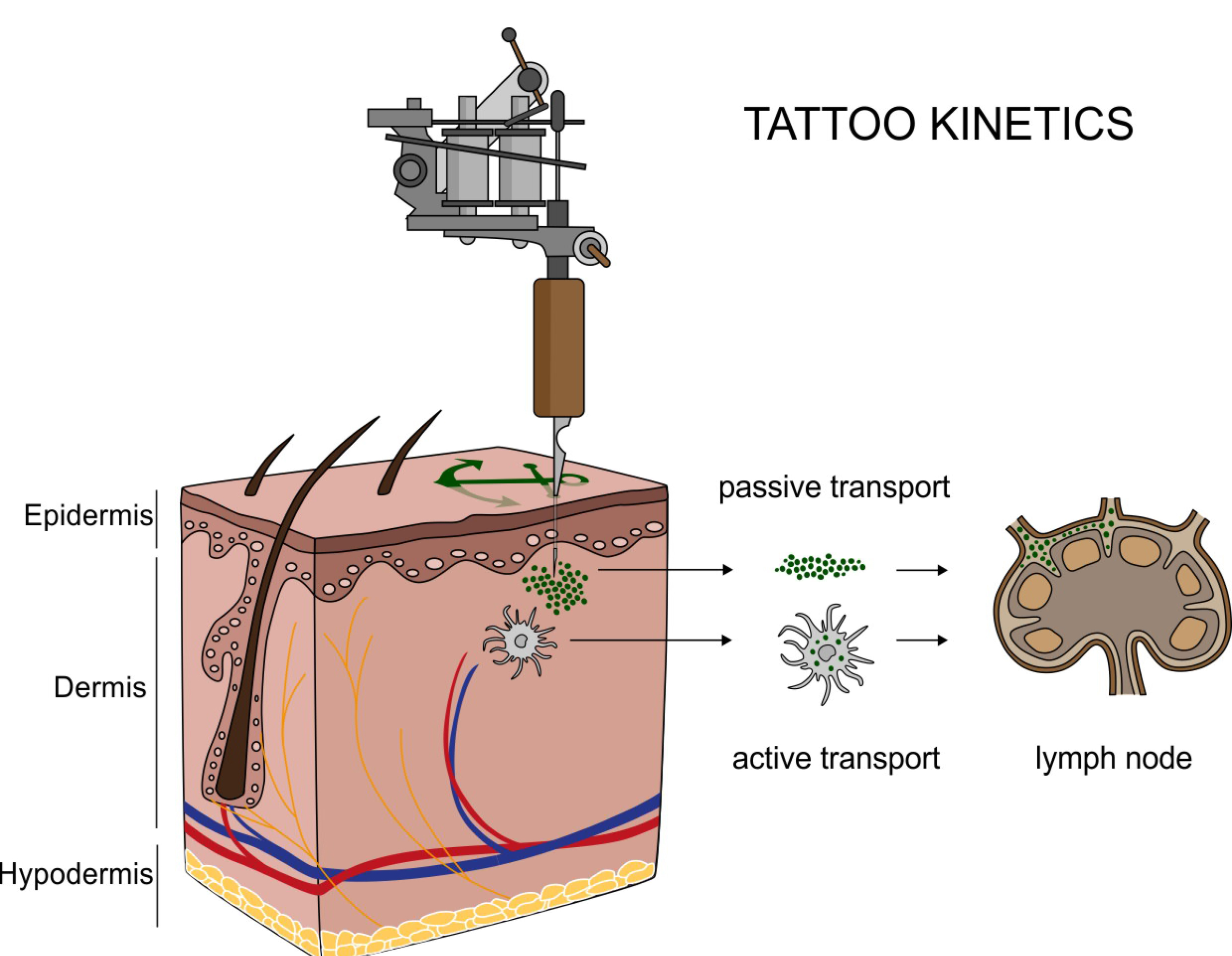
Translocation of tattoo particles from skin to lymph nodes. Upon injection of tattoo inks, particles can be either passively transported via blood and lymph fluids or phagocytized by immune cells and subsequently deposited in regional lymph nodes. After healing, particles are present in the dermis and in the sinusoids of the draining lymph nodes. Credits: C. Seim.
The hazards that potentially derive from tattoos were, until now, only investigated by chemical analysis of the inks and their degradation products in vitro. “We already knew that pigments from tattoos would travel to the lymph nodes because of visual evidence: the lymph nodes become tinted with the colour of the tattoo. It is the response of the body to clean the site of entrance of the tattoo. What we didn’t know is that they do it in a nano form, which implies that they may not have the same behaviour as the particles at a micro level. And that is the problem: we don’t know how nanoparticles react”, explains Bernhard Hesse, one of the two first authors of the study (together with Ines Schreiver, from the German Federal Institute for Risk Assessment) and ESRF visiting scientist.
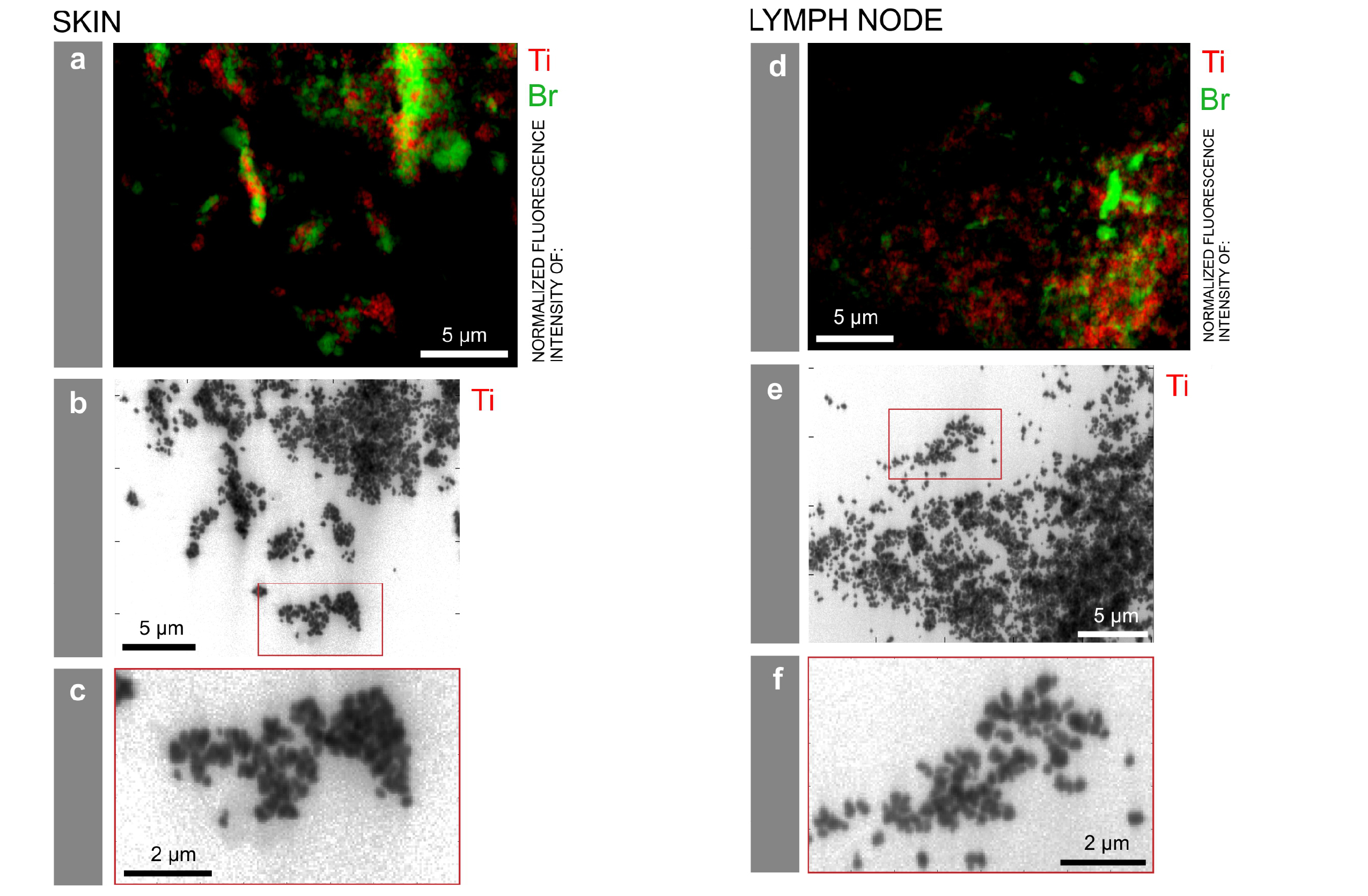
Particle mapping and size distribution of different tattoo pigment elements. a, d) Ti and the Br containing pigment phthalocyanine green 36 are located next to each other. b, e) Log scale mappings of Ti, Br and Fe in the same areas as displayed in a) and d) reveal primary particle sizes of different pigment species. c, f) Magnifications of the indicated areas in b) and e), respectively. Credits: C. Seim.
X-ray fluorescence measurements on ID21 allowed the team to locate titanium dioxide at the micro and nano range in the skin and the lymphatic environment. They found a broad range of particles with up to several micrometres in size in human skin, but only smaller (nano) particles transported to the lymph nodes. This can lead to the chronic enlargement of the lymph nodes and lifelong exposure. Scientists also used the technique of Fourier transform infrared spectroscopy to assess biomolecular changes in the tissues in the proximity of the tattoo particles.
Ines Schreiver doing experiments on ID16B with Julie Villanova. Credits: B. Hesse.
Altogether the scientists report strong evidence for both migration and long-term deposition of toxic elements and tattoo pigments as well as for conformational alterations of biomolecules that are sometimes linked to cutaneous adversities upon tattooing.
Then next step for the team is to inspect further samples of patients with adverse effects in their tattoos in order to find links with chemical and structural properties of the pigments used to create these tattoos.
Here’s a link to and a citation for the paper,
Synchrotron-based ν-XRF mapping and μ-FTIR microscopy enable to look into the fate and effects of tattoo pigments in human skin by Ines Schreiver, Bernhard Hesse, Christian Seim, Hiram Castillo-Michel, Julie Villanova, Peter Laux, Nadine Dreiack, Randolf Penning, Remi Tucoulou, Marine Cotte, & Andreas Luch. Scientific Reports 7, Article number: 11395 (2017) doi:10.1038/s41598-017-11721-z Published online: 12 September 2017
This paper is open access.