As I understand it, the big deal is that A*STAR (Singapore’s Agency for Science, Rechnology and Research) scientists have found a way to make a single molecule probe do the work of a two-molecule probe when imaging tumours. From a July 29, 2015 news item on Nanowerk (Note: A link has been removed),
An organic dye that can light up cancer cells for two powerful imaging techniques providing complementary diagnostic information has been developed and successfully tested in mice by A*STAR researchers (“Single Molecule with Dual Function on Nanogold: Biofunctionalized Construct for In Vivo Photoacoustic Imaging and SERS Biosensing”).
A July 29, 2015 A*STAR news release, which originated the news item, describes the currently used multimodal imaging technique and provides details about the new single molecule technique,
Imaging tumors is vitally important for cancer research, but each imaging technique has its own limitations for studying cancer in living organisms. To overcome the limitations of individual techniques, researchers typically employ a combination of various imaging methods — a practice known as multimodal imaging. In this way, they can obtain complementary information and hence a more complete picture of cancer.
Two very effective methods for imaging tumors are photoacoustic imaging and surface-enhanced Raman scattering (SERS). Photoacoustic imaging can image deep tissue with a good resolution, whereas SERS detects miniscule amounts of a target molecule. To simultaneously use both photoacoustic imaging and SERS, a probe must produce signals for both imaging modalities.
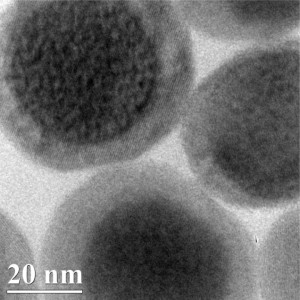
In multimodal imaging, researchers typically combine probes for each imaging modality into a single two-molecule probe. However, the teams of Malini Olivo at the A*STAR Singapore Bioimaging Consortium and Bin Liu at the A*STAR Institute of Materials Research and Engineering, along with overseas collaborator Ben Zhong Tang from the Hong Kong University of Science and Technology, adopted a different approach — they developed single-molecule probes that can be used for both photoacoustic imaging and SERS. The probes are based on organic cyanine dyes that absorb near-infrared light, which has the advantage of being able to deeply penetrate tissue, enabling tumors deep within the body to be imaged.
Once the team had verified that the probes worked for both imaging modalities, they optimized the performances of the probes by adding gold nanoparticles to them to amplify the SERS signal and by encapsulating them in the polymer polyethylene glycol to stabilize their structures.
The researchers then deployed these optimized probes in live mice. By functionalizing the probes with an antibody that recognizes a tumor cell-surface protein, they were able to use them to target tumors. The scientists found that, in photoacoustic imaging, the tumor-targeted probes produced signals that were roughly three times stronger than those of unmodified probes. Using SERS, the team was also able to monitor the concentrations of the probes in the tumor, spleen and liver in real time with a high degree of sensitivity.
U. S. Dinish, a senior scientist in Olivo’s group, recalls the team’s “surprise at the sensitivity and potential of the nanoconstruct.” He anticipates that the probe could be used to guide surgical removal of tumors.
Here’s a link to and a citation for the paper,
Single Molecule with Dual Function on Nanogold: Biofunctionalized Construct for In Vivo Photoacoustic Imaging and SERS Biosensing by U. S. Dinish, Zhegang Song, Chris Jun Hui Ho, Ghayathri Balasundaram, Amalina Binte Ebrahim Attia, Xianmao Lu, Ben Zhong Tang, Bin Liu, and Malini Olivo. Advanced Functional Materials, Vol 25 Issue 15
pages 2316–2325, April 15, 2015 DOI: 10.1002/adfm.201404341 Article first published online: 11 MAR 2015© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
This paper is behind a paywall.