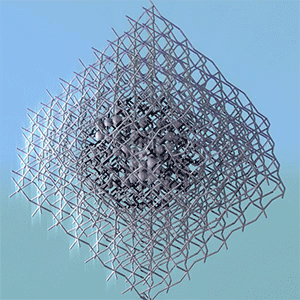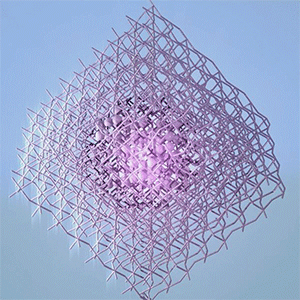
Researchers at the University of British Columbia (UBC; Canada) have announced a startling revelation according to an Oct. 6, 2015 news item on ScienceDaily,
As the push for thinner and faster electronics continues, a new finding by University of British Columbia scientists could help inform the design of the next generation of cheaper, more efficient devices.
The work, published this week in Nature Communications, details how electronic properties at the edges of organic molecular systems differ from the rest of the material.
An Oct. 6, 2015 UBC news release on EurekAlert, which originated the news item, expands on the theme,
Organic [as in carbon-based] materials–plastics–are of great interest for use in solar panels, light emitting diodes and transistors. They’re low-cost, light, and take less energy to produce than silicon. Interfaces–where one type of material meets another–play a key role in the functionality of all these devices.
“We found that the polarization-induced energy level shifts from the edge of these materials to the interior are significant, and can’t be neglected when designing components,” says UBC PhD researcher Katherine Cochrane, lead author of the paper.
‘While we were expecting some differences, we were surprised by the size of the effect and that it occurred on the scale of a single molecule,” adds UBC researcher Sarah Burke, an expert on nanoscale electronic and optoelectronic materials and author on the paper.
The researchers looked at ‘nano-islands’ of clustered organic molecules. The molecules were deposited on a silver crystal coated with an ultra-thin layer of salt only two atoms deep. The salt is an insulator and prevents electrons in the organic molecules from interacting with those in the silver–the researchers wanted to isolate the interactions of the molecules.
Not only did the molecules at the edge of the nano-islands have very different properties than in the middle, the variation in properties depended on the position and orientation of other molecules nearby.
The researchers, part of UBC’s Quantum Matter Institute, used a simple, analytical model to explain the differences which can be extended to predict interface properties in much more complex systems, like those encountered in a real device.
“Herbert Kroemer said in his Nobel Lecture that ‘The interface is the device’ and it’s equally true for organic materials,” says Burke. [emphasis mine] “The differences we’ve seen at the edges of molecular clusters highlights one effect that we’ll need to consider as we design new materials for these devices, but likely they are many more surprises waiting to be discovered.”
Cochrane and colleagues plan to keep looking at what happens at interfaces in these materials and to work with materials chemists to guide the design rules for the structure and electronic properties of future devices.
Methods
The experiment was performed at UBC’s state-of-the-art Laboratory for Atomic Imaging Research, which features three specially designed ultra-quiet rooms that allow the instruments to sit in complete silence, totally still, to perform their delicate measurements. This allowed the researchers to take dense data sets with a tool called a scanning tunnelling microscope (STM) that showed them the energy levels in real-space on the scale of single atoms.
Here’s a link to and a citation for the paper,
Pronounced polarization-induced energy level shifts at boundaries of organic semiconductor nanostructures by K. A. Cochrane, A. Schiffrin, T. S. Roussy, M. Capsoni, & S. A. Burke. Nature Communications 6, Article number: 8312 doi:10.1038/ncomms9312 Published 06 October 2015
This paper is open access. Yes, I borrowed from Nobel Laureate, Herbert Kroemer for the headline. As Woody Guthrie (legendary American folksinger) once said, more or less, “Only steal from the best.”