Two research groups are working to the same end where bone marrow is concerned, encourage bone cell growth, but they are using different strategies.
University of British Columbia and McMaster University (Canada)

The samples look a little like teeth, don’t they?
Before diving into the research news, there’s a terminology issue that should be noted as you’ll see when you read the news/press releases. Nanocrystal cellulose/nanocrystalline cellulose (NCC) is a term coined by Canadian researchers. Since those early day, most researchers, internationally, have adopted the term cellulose nanocrystals (CNC) as the standard term. It fits better with the naming conventions for other nnanocellulose materials such as cellulose nanofibrils, etc. By the way, a Canadian company (CelluForce) that produces CNC retained the term nanocrystalline cellulose (NCC) as a trademark for the product, CelluForce NCC®.
For anyone not familiar with aerogels, what the University of British Columbia (UBC) and McMaster University researchers are developing, are also popularly known known as ‘frozen smoke’ (see the Aerogel Wikipedia entry for more).
A March 19, 2019 news item on ScienceDaily announces the research,
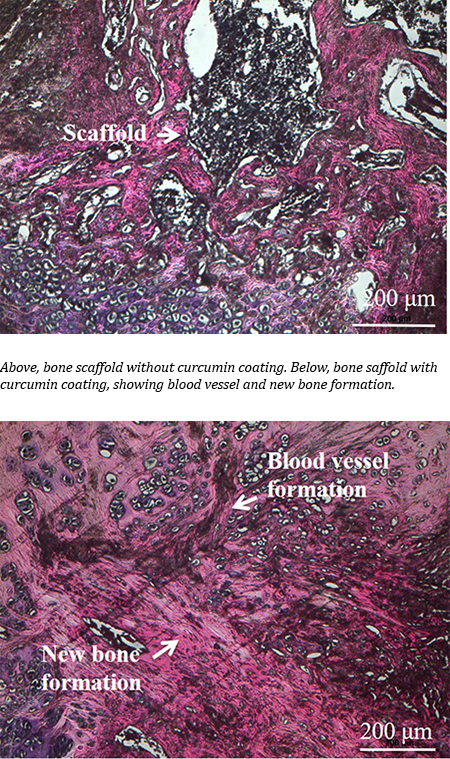
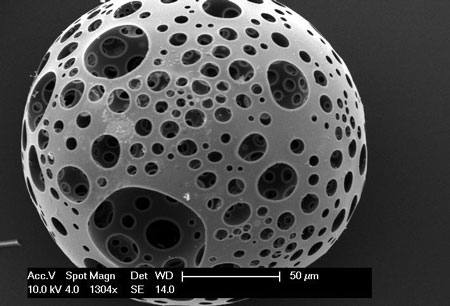
Researchers from the University of British Columbia and McMaster University have developed what could be the bone implant material of the future: an airy, foamlike substance that can be injected into the body and provide scaffolding for the growth of new bone.
It’s made by treating nanocrystals derived from plant cellulose so that they link up and form a strong but lightweight sponge — technically speaking, an aerogel — that can compress or expand as needed to completely fill out a bone cavity.
A March 19, 2019 UBC news release (also on EurekAlert), which originated the news item, describes the research in more detail,
“Most bone graft or implants are made of hard, brittle ceramic that doesn’t always conform to the shape of the hole, and those gaps can lead to poor growth of the bone and implant failure,” said study author Daniel Osorio, a PhD student in chemical engineering at McMaster. “We created this cellulose nanocrystal aerogel as a more effective alternative to these synthetic materials.”
For their research, the team worked with two groups of rats, with the first group receiving the aerogel implants and the second group receiving none. Results showed that the group with implants saw 33 per cent more bone growth at the three-week mark and 50 per cent more bone growth at the 12-week mark, compared to the controls.
“These findings show, for the first time in a lab setting, that a cellulose nanocrystal aerogel can support new bone growth,” said study co-author Emily Cranston, a professor of wood science and chemical and biological engineering who holds the President’s Excellence Chair in Forest Bio-products at UBC. She added that the implant should break down into non-toxic components in the body as the bone starts to heal.
The innovation can potentially fill a niche in the $2-billion bone graft market in North America, said study co-author Kathryn Grandfield, a professor of materials science and engineering, and biomedical engineering at McMaster who supervised the work.
“We can see this aerogel being used for a number of applications including dental implants and spinal and joint replacement surgeries,” said Grandfield. “And it will be economical because the raw material, the nanocellulose, is already being produced in commercial quantities.”
The researchers say it will be some time before the aerogel makes it out of the lab and into the operating room.
“This summer, we will study the mechanisms between the bone and implant that lead to bone growth,” said Grandfield. “We’ll also look at how the implant degrades using advanced microscopes. After that, more biological testing will be required before it is ready for clinical trials.”
Here’s a link to and a citation for the paper,
Cross-linked cellulose nanocrystal aerogels as viable bone tissue scaffolds by Daniel A. Osorio, Bryan E. J. Lee, Jacek M. Kwiecien, Xiaoyue Wang, Iflah Shahid, Ariana L. Hurley, Emily D. Cranston and Kathryn Grandfield. Acta Biomaterialia Volume 87, 15 March 2019, Pages 152-165 DOI: https://doi.org/10.1016/j.actbio.2019.01.049
This paper is behind a paywall
Now for the Russian team.
National University of Science and Technology “MISIS” (formerly part of the Moscow Mining Academy)
These scientists have adopted a different strategy as you’ll see in the March 19, 2019 news item on Nanwerk, which, coincidentally, was published on the same day as the Canadian research,
Scientists from the National University of Science and Technology “MISIS” developed a nanomaterial, which will be able to rstore the internal structure of bones damaged due to osteoporosis and osteomyelitis. A special bioactive coating of the material helped to increase the rate of division of bone cells by 3 times. In the future, it can allow to abandon bone marrow transplantation and patients will no longer need to wait for suitable donor material.
A March 19, 2019 National University of Science and Technology (MISIS) press release (also on EurekAlert), which originated the news item, provides detail about the impetus for the research and the technique being developed,
Such diseases as osteoporosis and osteomyelitis cause irreversible degenerative changes in the bone structure. Such diseases require serious complex treatment and surgery and transplantation of the destroyed bone marrow in severe stages. Donor material should have a number of compatibility indicators and even close relationship with the donor cannot guarantee full compatibility.
Research group from the National University of Science and Technology “MISIS” (NUST MISIS), led by Anton Manakhov (Laboratory for Inorganic Nanomaterials) developed material that will allow to restore damaged internal bone structure without bone marrow transplantation.
It is based on nanofibers of polycaprolactone, which is biocompatible self-dissolvable material. Earlier, the same research group has already worked with this material: by adding antibiotics to the nanofibers, scientists have managed to create non-changeable healing bandages.“If we want the implant to take, not only biocompatibility is needed, but also activation of the natural cell growth on the surface of the material. Polycaprolactone as such is a hydrophobic material, meaning, and cells feel uncomfortable on its surface. They gather on the smooth surface and divide extremely slow”, Elizaveta Permyakova, one of the co-authors and researcher at NUST MISIS Laboratory for Inorganic Nanomaterials, explains.
To increase the hydrophilicity of the material, a thin layer of bioactive film consisting of titanium, calcium, phosphorus, carbon, oxygen and nitrogen (TiCaPCON) was deposited on it. The structure of nanofibers identical to the cell surface was preserved. These films, when immersed in a special salt medium, which chemical composition is identical to human blood plasma, are able to form on its surface a special layer of calcium and phosphorus, which in natural conditions forms the main part of the bone. Due to the chemical similarity and the structure of nanofibers, new bone tissue begins to grow rapidly on this layer. Most importantly, polycaprolactone nanofibers dissolve, having fulfilled their functions. Only new “native” tissue remains in the bone.
In the experimental part of the study, the researchers compared the rate of division of osteoblastic bone cells on the surface of the modified and unmodified material. It was found that the modified material TiCaPCON has a high hydrophilicity. In contrast to the unmodified material, the cells on its surface felt clearly more comfortable, and divided three times faster.
According to scientists, such results open up great prospects for further work with modified polycaprolactone nanofibers as an alternative to bone marrow transplantation.
Here’s a link to and a citation for the paper,
Bioactive TiCaPCON-coated PCL nanofibers as a promising material for bone tissue engineering by Anton Manakhov, Elizaveta S. Permyakova, Sergey Ershov, Alexander Sheveyko, Andrey Kovalskii, Josef Polčák, Irina Y. Zhitnyak, Natalia A. Gloushankova, Lenka Zajíčková, Dmitry V. Shtansky. Applied Surface Science Volume 479, 15 June 2019, Pages 796-802 DOI: https://doi.org/10.1016/j.apsusc.2019.02.163
This paper is behind a paywall.