it seems Australian researchers are working hard to find ways of removing microplastics from water. I have two items, first, a November 29, 2022 news item on Nanowerk announces some of the latest work,
Researchers at RMIT University have found an innovative way to rapidly remove hazardous microplastics from water using magnets.
Lead researcher Professor Nicky Eshtiaghi said existing methods could take days to remove microplastics from water, while their cheap and sustainable invention achieves better results in just one hour.
The team says they have developed adsorbents, in the form of a powder, that remove microplastics 1,000 times smaller than those currently detectable by existing wastewater treatment plants.
The researchers have successfully tested the adsorbents in the lab, and they plan to engage with industry to further develop the innovation to remove microplastics from waterways.
…
A November 30, 2022 RMIT University press release, which originated the news item, provides more technical detail about the work,
“The nano-pillar structure we’ve engineered to remove this pollution, which is impossible to see but very harmful to the environment, is recycled from waste and can be used multiple times,” said Eshtiaghi from RMIT’s School of Environmental and Chemical Engineering.
“This is a big win for the environment and the circular economy.”
How does this innovation work?
The researchers have developed an adsorbent using nanomaterials that they can mix into water to attract microplastics and dissolved pollutants.
Muhammad Haris, the first author and PhD candidate from RMIT’s School of Environmental and Chemical Engineering, said the nanomaterials contained iron, which enabled the team to use magnets to easily separate the microplastics and pollutants from the water.
“This whole process takes one hour, compared to other inventions taking days,” he said.
Co-lead researcher Dr Nasir Mahmood said the nano-pillar structured material was designed to attract microplastics without creating any secondary pollutants or carbon footprints.
“The adsorbent is prepared with special surface properties so that it can effectively and simultaneously remove both microplastics and dissolved pollutants from water,” said Mahmood from Applied Chemistry and Environmental Science at RMIT.
“Microplastics smaller than 5 millimetres, which can take up to 450 years to degrade, are not detectable and removable through conventional treatment systems, resulting in millions of tonnes being released into the sea every year. This is not only harmful for aquatic life, but also has significant negative impacts on human health.”
The team received scientific and technical support from the Microscopy and Microanalysis Facility and the Micro Nano Research Facility, part of RMIT’s newly expanded Advanced Manufacturing Precinct, to complete their research.
What are the next steps?
Developing a cost-effective way to overcome these signficant challenges posed by microplastics was critical, Eshtiaghi said.
“Our powder additive can remove microplastics that are 1,000 times smaller than those that are currently detectable by existing wastewater treatment plants,” she said.
“We are looking for industrial collaborators to take our invention to the next steps, where we will be looking at its application in wastewater treatment plants.”
Eshtiaghi and her colleagues have worked with various water utilities across Australia, including with Melbourne Water and Water Corporation in Perth on a recent Australian Research Council Linkage project to optimise sludge pumping systems.
Here’s a link to and a citation for the paper,
Self-assembly of C@FeO nanopillars on 2D-MOF for simultaneous removal of microplastic and dissolved contaminants from water by Muhammad Haris, Muhammad Waqas Khan, Ali Zavabeti, Nasir Mahmood and Nicky Eshtiaghi. Chemical Engineering Journal Available online 23 November 2022, 140390 DOI: https://doi.org/10.1016/j.cej.2022.140390
This paper is behind a paywall.
Back in 2019
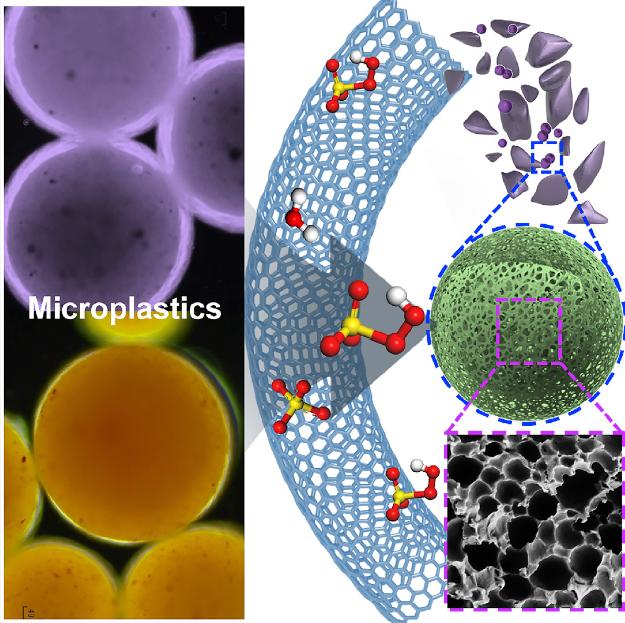
This July 31, 2019 Cell Press news release on EurekAlert announces a different approach, from an Australian team, to removing plastics from water,
Plastic waste that finds its way into oceans and rivers poses a global environmental threat with damaging health consequences for animals, humans, and ecosystems. Now, using tiny coil-shaped carbon-based magnets, researchers in Australia have developed a new approach to purging water sources of the microplastics that pollute them without harming nearby microorganisms. Their work appears July 31 in the journal Matter.
“Microplastics adsorb organic and metal contaminants as they travel through water and release these hazardous substances into aquatic organisms when eaten, causing them to accumulate all the way up the food chain” says senior author Shaobin Wang, a professor of chemical engineering at the University of Adelaide (Australia). “Carbon nanosprings are strong and stable enough to break these microplastics down into compounds that do not pose such a threat to the marine ecosystem.”
Although often invisible to the naked eye, microplastics are ubiquitous pollutants. Some, such as the exfoliating beads found in popular cosmetics, are simply too small to be filtered out during industrial water treatment. Others are produced indirectly, when larger debris like soda bottles or tires weather amid sun and sand.
To decompose the microplastics, the researchers had to generate short-lived chemicals called reactive oxygen species, which trigger chain reactions that chop the various long molecules that make up microplastics into tiny and harmless segments that dissolve in water. However, reactive oxygen species are often produced using heavy metals such as iron or cobalt, which are dangerous pollutants in their own right and thus unsuitable in an environmental context.
To get around this challenge, the researchers found a greener solution in the form of carbon nanotubes laced with nitrogen to help boost generation of reactive oxygen species. Shaped like springs, the carbon nanotube catalysts removed a significant fraction of microplastics in just eight hours while remaining stable themselves in the harsh oxidative conditions needed for microplastics breakdown. The coiled shape increases stability and maximises reactive surface area. As a bonus, by including a small amount of manganese, buried far from the surface of the nanotubes to prevent it from leaching into water, the minute springs became magnetic.
“Having magnetic nanotubes is particularly exciting because this makes it easy to collect them from real wastewater streams for repeated use in environmental remediation,” says Xiaoguang Duan, a chemical engineering research fellow at Adelaide who also co-led the project.
As no two microplastics are chemically quite the same, the researchers’ next steps will center on ensuring that the nanosprings work on microplastics of different compositions, shapes and origins. They also intend to continue to rigorously confirm the non-toxicity of any chemical compounds occurring as intermediates or by-products during microplastics decomposition.
The researchers also say that those intermediates and byproducts could be harnessed as an energy source for microorganisms that the polluting plastics currently plague. “If plastic contaminants can be repurposed as food for algae growth, it will be a triumph for using biotechnology to solve environmental problems in ways that are both green and cost efficient,” Wang says.
Here’s a link to and a citation for the paper,
Degradation of Cosmetic Microplastics via Functionalized Carbon Nanosprings by Jian Kang, Li Zhou, Xiaoguang Duan, Hongqi Sun, Zhimin Ao, Shaobin Wang. Matter Volume 1, Issue 3, 4 September 2019, Pages 745-758 DOI: https://doi.org/10.1016/j.matt.2019.06.004
This paper is open access.
Comments
I’m glad to see this work and as for which approach might be preferable, I don’t know if there’s a clear winner. The 2022 work removes both microplastics and pollutants in one hour! An impressive feat, which leaves us with microplastics and pollutants to deal with. By contrast , the 2019 work transforms the microplastics into materials that don’t pose harm to the aquatic environment. Great although it takes eight hours. I wish the best for all the researchers working on this microplastics problem.