A Jan. 21, 2016 news item on ScienceDaily announces a brand new technique,
For the first time, scientists have been able to weave a material at molecular level. The research is led by University of California Berkeley, in cooperation with Stockholm University. …
A Jan. 21, 2016 Stockholm University press release, which originated the news item, provides more information,
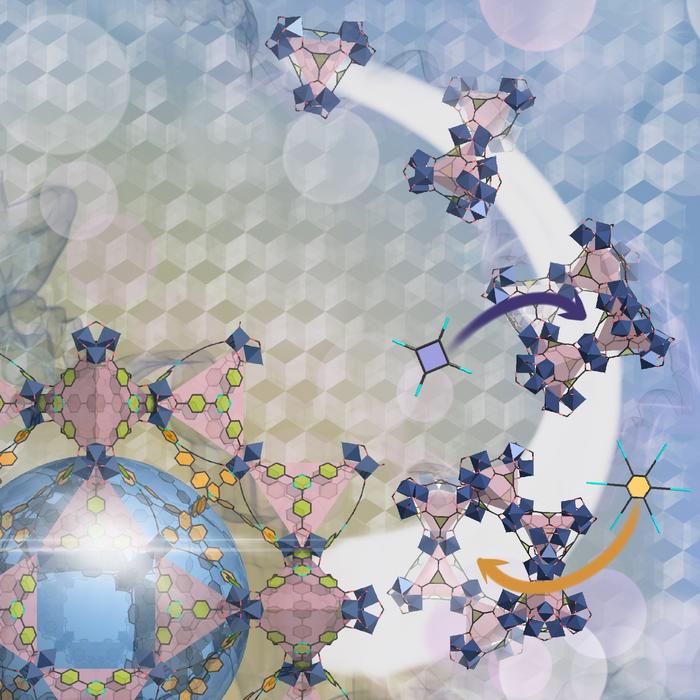
Weaving is a well-known way of making fabric, but has until now never been used at the molecular level. Scientists have now been able to weave organic threads into a three-dimensional material, using copper as a template. The new material is a COF, covalent organic framework, and is named COF-505. The copper ions can be removed and added without changing the underlying structure, and at the same time the elasticity can be reversibly changed.
– It almost looks like a molecular version of the Vikings chain-armour. The material is very flexible, says Peter Oleynikov, researcher at the Department of Materials and Environmental Chemistry at Stockholm University.
COF’s are like MOF’s porous three-dimensional crystals with a very large internal surface that can adsorb and store enormous quantities of molecules. A potential application is capture and storage of carbon dioxide, or using COF’s as a catalyst to make useful molecules from carbon dioxide.
Complex structure determined in Stockholm
The research is led by Professor Omar Yaghi at University of California Berkeley. At Stockholm University Professor Osamu Terasaki, PhD Student Yanhang Ma and Researcher Peter Oleynikov have contributed to determine the structure of COF-505 at atomic level from a nano-crystal, using electron crystallography methods.
– It is a difficult, complicated structure and it was very demanding to resolve. We’ve spent lot of time and efforts on the structure solution. Now we know exactly where the copper is and we can also replace the metal. This opens up many possibilities to make other materials, says Yanhang Ma, PhD Student at the Department of Materials and Environmental Chemistry at Stockholm University.
Another of the collaborating institutions, US Department of Energy Lawrence Berkeley National Laboratory issued a Jan. 21, 2016 news release on EurekAlert, providing a different perspective and some additional details,
There are many different ways to make nanomaterials but weaving, the oldest and most enduring method of making fabrics, has not been one of them – until now. An international collaboration led by scientists at the U.S. Department of Energy (DOE)’s Lawrence Berkeley National Laboratory (Berkeley Lab) and the University of California (UC) Berkeley, has woven the first three-dimensional covalent organic frameworks (COFs) from helical organic threads. The woven COFs display significant advantages in structural flexibility, resiliency and reversibility over previous COFs – materials that are highly prized for their potential to capture and store carbon dioxide then convert it into valuable chemical products.
…
“Weaving in chemistry has been long sought after and is unknown in biology,” Yaghi says [Omar Yaghi, chemist who holds joint appointments with Berkeley Lab’s Materials Sciences Division and UC Berkeley’s Chemistry Department and is the co-director of the Kavli Energy NanoScience Institute {Kavli-ENSI}]. “However, we have found a way of weaving organic threads that enables us to design and make complex two- and three-dimensional organic extended structures.”
…
COFs and their cousin materials, metal organic frameworks (MOFs), are porous three-dimensional crystals with extraordinarily large internal surface areas that can absorb and store enormous quantities of targeted molecules. Invented by Yaghi, COFs and MOFs consist of molecules (organics for COFs and metal-organics for MOFs) that are stitched into large and extended netlike frameworks whose structures are held together by strong chemical bonds. Such frameworks show great promise for, among other applications, carbon sequestration.
Through another technique developed by Yaghi, called “reticular chemistry,” these frameworks can also be embedded with catalysts to carry out desired functions: for example, reducing carbon dioxide into carbon monoxide, which serves as a primary building block for a wide range of chemical products including fuels, pharmaceuticals and plastics.
In this latest study, Yaghi and his collaborators used a copper(I) complex as a template for bringing threads of the organic compound “phenanthroline” into a woven pattern to produce an immine-based framework they dubbed COF-505. Through X-ray and electron diffraction characterizations, the researchers discovered that the copper(I) ions can be reversibly removed or restored to COF-505 without changing its woven structure. Demetalation of the COF resulted in a tenfold increase in its elasticity and remetalation restored the COF to its original stiffness.
“That our system can switch between two states of elasticity reversibly by a simple operation, the first such demonstration in an extended chemical structure, means that cycling between these states can be done repeatedly without degrading or altering the structure,” Yaghi says. “Based on these results, it is easy to imagine the creation of molecular cloths that combine unusual resiliency, strength, flexibility and chemical variability in one material.”
Yaghi says that MOFs can also be woven as can all structures based on netlike frameworks. In addition, these woven structures can also be made as nanoparticles or polymers, which means they can be fabricated into thin films and electronic devices.
“Our weaving technique allows long threads of covalently linked molecules to cross at regular intervals,” Yaghi says. “These crossings serve as points of registry, so that the threads have many degrees of freedom to move away from and back to such points without collapsing the overall structure, a boon to making materials with exceptional mechanical properties and dynamics.”
###
This research was primarily supported by BASF (Germany) and King Abdulaziz City for Science and Technology (KACST).
It’s unusual that neither Stockholm University not the Lawrence Berkeley National Laboratory list all of the institutions involved. To get a sense of this international collaboration’s size, I’m going to list them,
-
1Department of Chemistry, University of California, Berkeley, Materials Sciences Division, Lawrence Berkeley National Laboratory, and Kavli Energy NanoSciences Institute, Berkeley, CA 94720, USA.
-
2Department of Materials and Environmental Chemistry, Stockholm University, SE-10691 Stockholm, Sweden.
-
3Department of New Architectures in Materials Chemistry, Materials Science Institute of Madrid, Consejo Superior de Investigaciones Científicas, Madrid 28049, Spain.
- 4Nanomaterials Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba 305-8565, Japan.
- 5NSF Nanoscale Science and Engineering Center (NSEC), University of California at Berkeley, 3112 Etcheverry Hall, Berkeley, CA 94720, USA.
- 6Advanced Light Source, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA.
- 7King Abdulaziz City of Science and Technology, Post Office Box 6086, Riyadh 11442, Saudi Arabia.
- 8Material Sciences Division, Lawrence Berkeley National Laboratory, 1 Cyclotron Road, Berkeley, CA 94720, USA.
- 9School of Physical Science and Technology, ShanghaiTech University, Shanghai 201210, China.
Given that some of the money came from a German company, I’m surprised not one German institution was involved.
Here’s a link to and citation for the paper,
Weaving of organic threads into a crystalline covalent organic framework by Yuzhong Liu, Yanhang Ma, Yingbo Zhao, Xixi Sun, Felipe Gándara, Hiroyasu Furukawa, Zheng Liu, Hanyu Zhu, Chenhui Zhu, Kazutomo Suenaga, Peter Oleynikov, Ahmad S. Alshammari, Xiang Zhang, Osamu Terasaki, Omar M. Yaghi. Science 22 Jan 2016: Vol. 351, Issue 6271, pp. 365-369 DOI: 10.1126/science.aad4011
This paper is behind a paywall.