While watching this video I started wondering if they were testing their research on students but that’s not the case; these wearable patches were tested on porcine (pig) skin, which is quite similar to human skin, Note: They tested a B vitamin called niacinamide so, it’s highly unlikely the pigs suffered from it,
An April 20, 2023 news item on ScienceDaily announces the research into using ultrasonic waves for drug delivery,
The skin is an appealing route for drug delivery because it allows drugs to go directly to the site where they’re needed, which could be useful for wound healing, pain relief, or other medical and cosmetic applications. However, delivering drugs through the skin is difficult because the tough outer layer of the skin prevents most small molecules from passing through it.
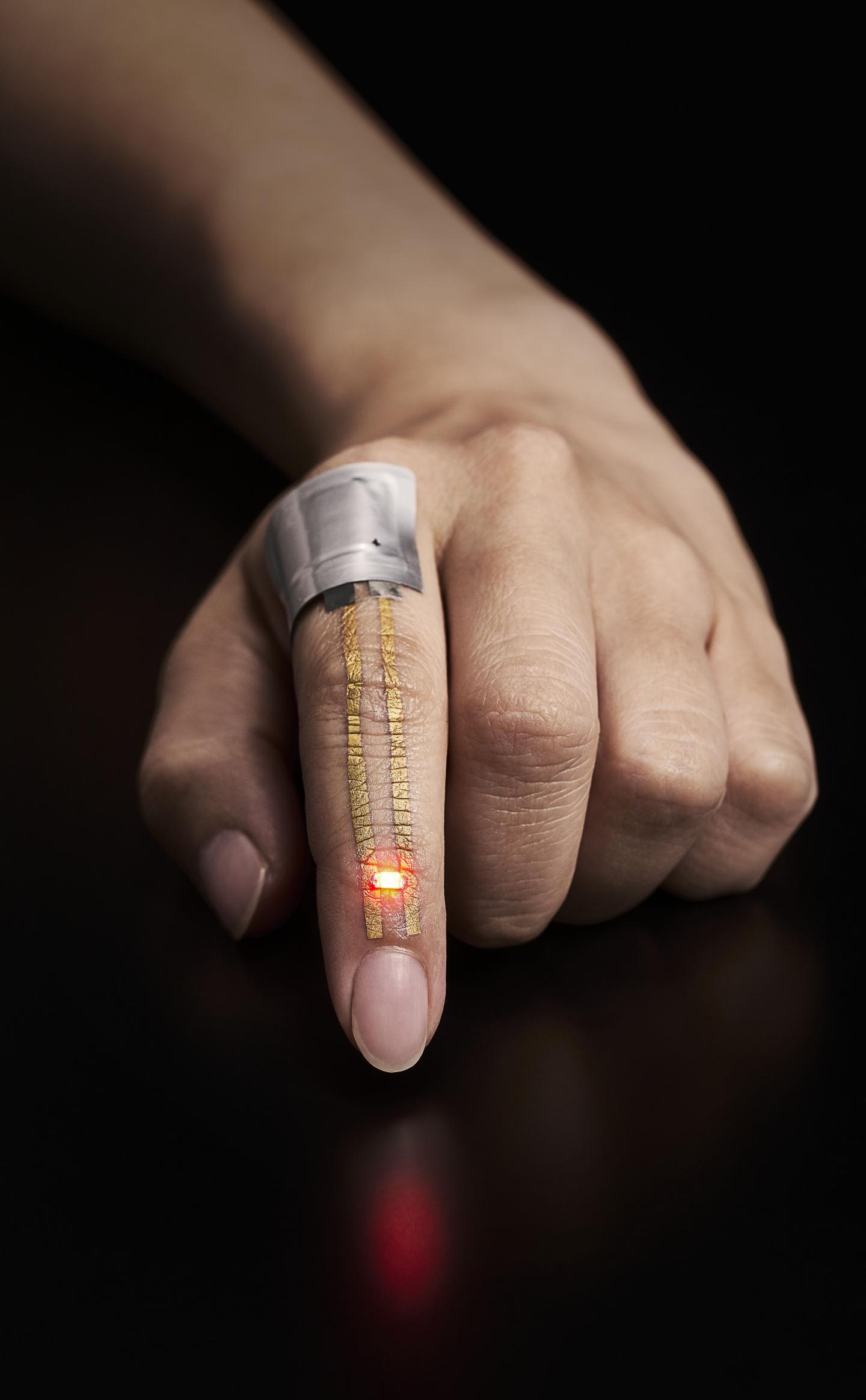
In hopes of making it easier to deliver drugs through the skin, MIT [Massachusetts Institute of Technology] researchers have developed a wearable patch that applies painless ultrasonic waves to the skin, creating tiny channels that drugs can pass through. This approach could lend itself to delivery of treatments for a variety of skin conditions, and could also be adapted to deliver hormones, muscle relaxants, and other drugs, the researchers say.
…
An April 20, 2023 Massachusetts Institute of Technology (MIT) news release (also on EurekAlert), which originated the news item, provides technical details about the research, Note: A link has been removed,
“The ease-of-use and high-repeatability offered by this system provides a game-changing alternative to patients and consumers suffering from skin conditions and premature skin aging,” says Canan Dagdeviren, an associate professor in MIT’s Media Lab and the senior author of the study. “Delivering drugs this way could offer less systemic toxicity and is more local, comfortable, and controllable.”
MIT research assistants Chia-Chen Yu and Aastha Shah are the lead authors of the paper, which appears in Advanced Materials, as part of the journal’s “Rising Stars” series, which showcases the outstanding work of researchers in the early stages of their independent careers. Other MIT authors include Research Assistant Colin Marcus and postdoc Md Osman Goni Nayeem. Nikta Amiri, Amit Kumar Bhayadia, and Amin Karami of the University of Buffalo are also authors of the paper.
A boost from sound waves
The researchers began this project as an exploration of alternative ways to deliver drugs. Most drugs are delivered orally or intravenously, but the skin is a route that could offer much more targeted drug delivery for certain applications.
“The main benefit with skin is that you bypass the whole gastrointestinal tract. With oral delivery, you have to deliver a much larger dose in order to account for the loss that you would have in the gastric system,” Shah says. “This is a much more targeted, focused modality of drug delivery.”
Ultrasound exposure has been shown to enhance the skin’s permeability to small-molecule drugs, but most of the existing techniques for performing this kind of drug delivery require bulky equipment. The MIT team wanted to come up with a way to perform this kind of transdermal drug delivery with a lightweight, wearable patch, which could make it easier to use for a variety of applications.
The device that they designed consists of a patch embedded with several disc-shaped piezoelectric transducers, which can convert electric currents into mechanical energy. Each disc is embedded in a polymeric cavity that contains the drug molecules dissolved in a liquid solution. When an electric current is applied to the piezoelectric elements, they generate pressure waves in the fluid, creating bubbles that burst against the skin. These bursting bubbles produce microjets of fluid that can penetrate through the skin’s tough outer layer, the stratum corneum.
“This works open the door to using vibrations to enhance drug delivery. There are several parameters that result in generation of different kinds of waveform patterns. Both mechanical and biological aspects of drug delivery can be improved by this new toolset,” Karami says.
The patch is made of PDMS, a silicone-based polymer that can adhere to the skin without tape. In this study, the researchers tested the device by delivering a B vitamin called niacinamide, an ingredient in many sunscreens and moisturizers.
In tests using pig skin, the researchers showed that when they delivered niacinamide using the ultrasound patch, the amount of drug that penetrated the skin was 26 times greater than the amount that could pass through the skin without ultrasonic assistance.
The researchers also compared the results from their new device to microneedling, a technique sometimes used for transdermal drug delivery, which involves puncturing the skin with miniature needles. The researchers found that their patch was able to deliver the same amount of niacinamide in 30 minutes that could be delivered with microneedles over a six-hour period.
Local delivery
With the current version of the device, drugs can penetrate a few millimeters into the skin, making this approach potentially useful for drugs that act locally within the skin. These could include niacinamide or vitamin C, which is used to treat age spots or other dark spots on the skin, or topical drugs used to heal burns.
With further modifications to increase the penetration depth, this technique could also be used for drugs that need to reach the bloodstream, such as caffeine, fentanyl, or lidocaine. Dagdeviren also envisions that this kind of patch could be useful for delivering hormones such as progesterone. In addition, the researchers are now exploring the possibility of implanting similar devices inside the body to deliver drugs to treat cancer or other diseases.
The researchers are also working on further optimizing the wearable patch, in hopes of testing it soon on human volunteers. They also plan to repeat the lab experiments they did in this study, with larger drug molecules.
“After we characterize the drug penetration profiles for much larger drugs, we would then see which candidates, like hormones or insulin, can be delivered using this technology, to provide a painless alternative for those who are currently bound to self-administer injections on a daily basis,” Shah says.
Here’s a link to and a citation for the paper,
A Conformable Ultrasound Patch for Cavitation-Enhanced Transdermal Cosmeceutical Delivery by Chia-Chen Yu, Aastha Shah, Nikta Amiri, Colin Marcus, Md Osman Goni Nayeem, Amit Kumar Bhayadia, Amin Karami, Canan Dagdeviren. Advanced Materials Volume35, Issue 23 June 8, 2023 2300066 DOI: https://doi.org/10.1002/adma.202300066 First published online: 19 March 2023
This paper is open access.


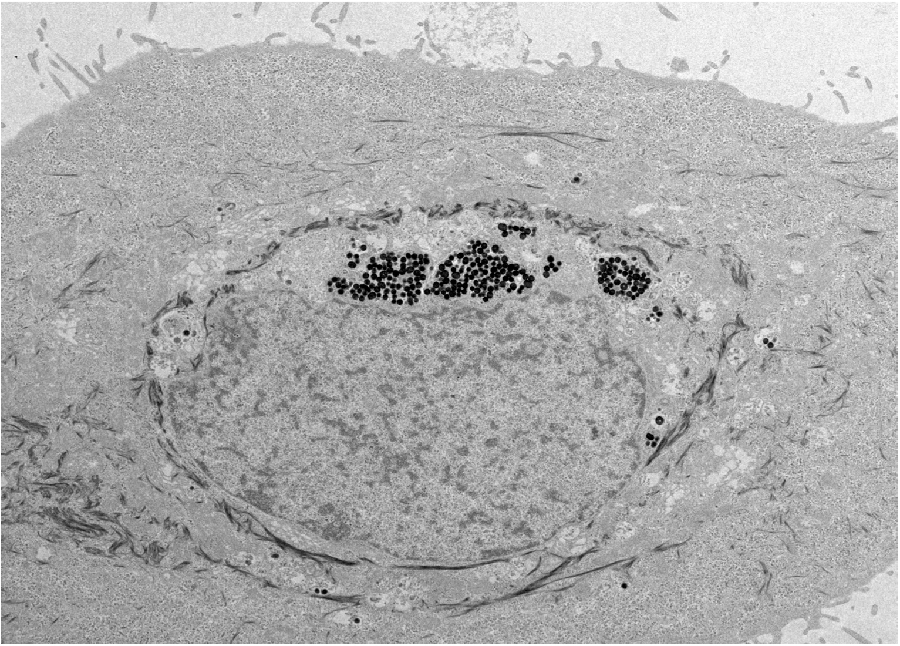 The scientists found that the synthetic nanoparticles were taken up in tissue culture by keratinocytes, the predominant cell type found in the epidermis, the outer layer of skin. Photo by Yuran Huang and Ying Jones/UC San Diego
The scientists found that the synthetic nanoparticles were taken up in tissue culture by keratinocytes, the predominant cell type found in the epidermis, the outer layer of skin. Photo by Yuran Huang and Ying Jones/UC San Diego


