
A March 9, 2023 news item on Nanowerk announces research into multicolour energy-saving coating/paint, so, this is a structural colour story, Note: Links have been removed,
University of Central Florida researcher Debashis Chanda, a professor in UCF’s NanoScience Technology Center, has drawn inspiration from butterflies to create the first environmentally friendly, large-scale and multicolor alternative to pigment-based colorants, which can contribute to energy-saving efforts and help reduce global warming.
…
A March 8, 2023 University of Central Florida (UCF) news release (also on EurekAlert) by Katrina Cabansay, which originated the news item, provides more context and more details,
“The range of colors and hues in the natural world are astonishing — from colorful flowers, birds and butterflies to underwater creatures like fish and cephalopods,” Chanda says. “Structural color serves as the primary color-generating mechanism in several extremely vivid species where geometrical arrangement of typically two colorless materials produces all colors. On the other hand, with manmade pigment, new molecules are needed for every color present.”
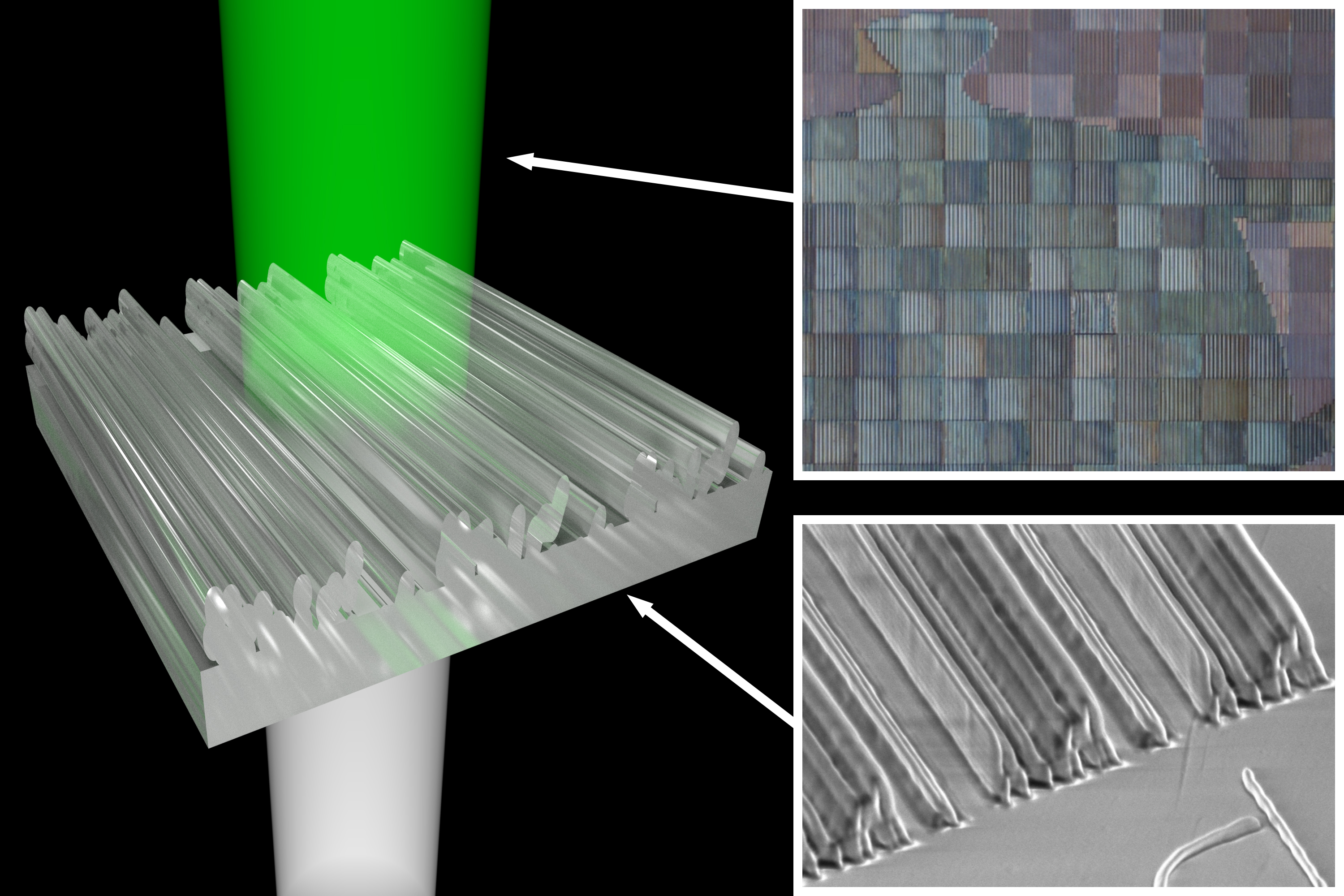
Based on such bio-inspirations, Chanda’s research group innovated a plasmonic paint, which utilizes nanoscale structural arrangement of colorless materials — aluminum and aluminum oxide — instead of pigments to create colors.
While pigment colorants control light absorption based on the electronic property of the pigment material and hence every color needs a new molecule, structural colorants control the way light is reflected, scattered or absorbed based purely on the geometrical arrangement of nanostructures.
Such structural colors are environmentally friendly as they only use metals and oxides, unlike present pigment-based colors that use artificially synthesized molecules.
The researchers have combined their structural color flakes with a commercial binder to form long-lasting paints of all colors.
“Normal color fades because pigment loses its ability to absorb photons,” Chanda says. “Here, we’re not limited by that phenomenon. Once we paint something with structural color, it should stay for centuries.”
Additionally, because plasmonic paint reflects the entire infrared spectrum, less heat is absorbed by the paint, resulting in the underneath surface staying 25 to 30 degrees Fahrenheit cooler than it would if it were covered with standard commercial paint, the researcher says.
“Over 10% of total electricity in the U.S. goes toward air conditioner usage,” Chanda says. “The temperature difference plasmonic paint promises would lead to significant energy savings. Using less electricity for cooling would also cut down carbon dioxide emissions, lessening global warming.”
Plasmonic paint is also extremely lightweight, the researcher says.
This is due to the paint’s large area-to-thickness ratio, with full coloration achieved at a paint thickness of only 150 nanometers, making it the lightest paint in the world, Chanda says.
The paint is so lightweight that only about 3 pounds of plasmonic paint could cover a Boeing 747, which normally requires more than 1,000 pounds of conventional paint, he says.
Chanda says his interest in structural color stems from the vibrancy of butterflies.
“As a kid, I always wanted to build a butterfly,” he says. “Color draws my interest.”
Future Research
Chanda says the next steps of the project include further exploration of the paint’s energy-saving aspects to improve its viability as commercial paint.
“The conventional pigment paint is made in big facilities where they can make hundreds of gallons of paint,” he says. “At this moment, unless we go through the scale-up process, it is still expensive to produce at an academic lab.”
“We need to bring something different like, non-toxicity, cooling effect, ultralight weight, to the table that other conventional paints can’t.” Chanda says.
Licensing Opportunity
For more information about licensing this technology, please visit the Inorganic Paint Pigment for Vivid Plasmonic Color technology sheet.
Researcher’s Credentials
Chanda has joint appointments in UCF’s NanoScience Technology Center, Department of Physics and College of Optics and Photonics. He received his doctorate in photonics from the University of Toronto and worked as a postdoctoral fellow at the University of Illinois at Urbana-Champaign. He joined UCF in Fall 2012.
Here’s a link to and a citation for the paper,
Ultralight plasmonic structural color paint by Pablo Cencillo-Abad, Daniel Franklin, Pamela Mastranzo-Ortega, Javier Sanchez-Mondragon, and Debashis Chanda. Science Advances 8 Mar 2023 Vol 9, Issue 10 DOI: 10.1126/sciadv.adf7207
This paper is open access.
Here’s the researcher with one of ‘his butterflies’ (I may be reading a little too much into this but it looks like he’s uncomfortable having his photo taken but game to do it for work that he’s proud of),





![Cobalt Blue Tarantula [downloaded from http://www.tarantulaguide.com/tarantula-pictures/cobalt-blue-tarantula-4/]](http://www.frogheart.ca/wp-content/uploads/2015/12/CobaltBlueTarantula.jpg)




