According to University of Portsmouth physicist, James Burriidge, determining how linguistic dialects form is a question for physics and mathematics. Here’s more about Burridge and his latest work on the topic from a July 24, 2017 University of Portsmouth press release (also on EurekAlert),
Language patterns could be predicted by simple laws of physics, a new study has found.
Dr James Burridge from the University of Portsmouth has published a theory using ideas from physics to predict where and how dialects occur.
He said: “If you want to know where you’ll find dialects and why, a lot can be predicted from the physics of bubbles and our tendency to copy others around us.
“Copying causes large dialect regions where one way of speaking dominates. Where dialect regions meet, you get surface tension. Surface tension causes oil and water to separate out into layers, and also causes small bubbles in a bubble bath to merge into bigger ones.
“The bubbles in the bath are like groups of people – they merge into the bigger bubbles because they want to fit in with their neighbours.
“When people speak and listen to each other, they have a tendency to conform to the patterns of speech they hear others using, and therefore align their dialects. Since people typically remain geographically local in their everyday lives, they tend to align with those nearby.”
Dr Burridge from the University’s department of mathematics departs from the existing approaches in studying dialects to formulate a theory of how country shape and population distribution play an important role in how dialect regions evolve.
Traditional dialectologists use the term ‘isogloss’ to describe a line on a map marking an area which has a distinct linguistic feature.
Dr Burridge said: “These isoglosses are like the edges of bubbles – the maths used to describe bubbles can also describe dialects.
“My model shows that dialects tend to move outwards from population centres, which explains why cities have their own dialects. Big cities like London and Birmingham are pushing on the walls of their own bubbles.
“This is why many dialects have a big city at their heart – the bigger the city, the greater this effect. It’s also why new ways of speaking often spread outwards from a large urban centre.
“If people live near a town or city, we assume they experience more frequent interactions with people from the city than with those living outside it, simply because there are more city dwellers to interact with.
His model also shows that language boundaries get smoother and straighter over time, which stabilises dialects.
Dr Burridge’s research is driven by a long-held interest in spatial patterns and the idea that humans and animal behaviour can evolve predictably. His research has been funded by the Leverhulme Trust.
Here’s an image illustrating language distribution in the UK<
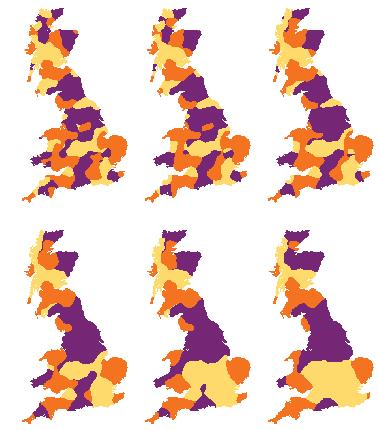
Caption: These maps show a simulation of three language variants that are initially distributed throughout Great Britain in a random pattern. As time passes (left to right), the boundaries between language variants tend to shorten in length. One can also see evidence of boundary lines fixing to river inlets and other coastal indentations. Credit: James Burridge, University of Portsmouth
Burridge has written an Aug. 2, 2017 essay for The Conversation which delves into the history of using physics and mathematics to understand social systems and further explains his own theory (Note: Links have been removed),
What do the physics of bubbles have in common with the way you and I speak? Not a lot, you might think. But my recently published research uses the physics of surface tension (the effect that determines the shape of bubbles) to explore language patterns – where and how dialects occur.
This connection between physical and social systems may seem surprising, but connections of this kind have a long history. The 19th century physicist Ludwig Boltzmann spent much of his life trying to explain how the physical world behaves based on some simple assumptions about the atoms from which it is made. His theories, which link atomic behaviour to the large scale properties of matter, are called “statistical mechanics”. At the time, there was considerable doubt that atoms even existed, so Boltzmann’s success is remarkable because the detailed properties of the systems he was studying were unknown.
The idea that details don’t matter when you are considering a very large number of interacting agents is tantalising for those interested in the collective behaviour of large groups of people. In fact, this idea can be traced back to another 19th century great, Leo Tolstoy, who argued in War and Peace:
“To elicit the laws of history we must leave aside kings, ministers, and generals, and select for study the homogeneous, infinitesimal elements which influence the masses.”
Mathematical history
Tolstoy was, in modern terms, advocating a statistical mechanics of history. But in what contexts will this approach work? If we are guided by what worked for Boltzmann, then the answer is quite simple. We need to look at phenomena which arise from large numbers of interactions between individuals rather than phenomena imposed from above by some mighty ruler or political movement.
To test a physical theory, one just needs a lab. But a mathematical historian must look for data that have already been collected, or can be extracted from existing sources. An ideal example is language dialects. For centuries, humans have been drawing maps of the spatial domains in which they live, creating records of their languages, and sometimes combining the two to create linguistic atlases. The geometrical picture which emerges is fascinating. As we travel around a country, the way that people use language, from their choices of words to their pronunciation of vowels, changes. Researchers quantify differences using “linguistic variables”.
For example, in 1950s England, the ulex shrub went by the name “gorse”, “furze”, “whim” or “broom” depending on where you were in the country. If we plot where these names are used on a map, we find large regions where one name is in common use, and comparatively narrow transition regions where the most common word changes. Linguists draw lines, called “isoglosses”, around the edges of regions where one word (or other linguistic variable) is common. As you approach an isogloss, you find people start to use a different word for the same thing.
A similar effect can be seen in sheets of magnetic metal where individual atoms behave like miniature magnets which want to line up with their neighbours. As a result, large regions appear in which the magnetic directions of all atoms are aligned. If we think of magnetic direction as an analogy for choice of linguistic variant – say up is “gorse” and down is “broom” – then aligning direction is like beginning to use the local word for ulex.
…
Linguistic maths
I made just one assumption about language evolution: that people tend to pick up ways of speaking which they hear in the geographical region where they spend most of their time. Typically, this region will be a few miles or tens of miles wide and centred on their home, but its shape may be skewed by the presence of a nearby city which they visit more often than the surrounding countryside.
…
For example, in 1950s England, the ulex shrub went by the name “gorse”, “furze”, “whim” or “broom” depending on where you were in the country. If we plot where these names are used on a map, we find large regions where one name is in common use, and comparatively narrow transition regions where the most common word changes. Linguists draw lines, called “isoglosses”, around the edges of regions where one word (or other linguistic variable) is common. As you approach an isogloss, you find people start to use a different word for the same thing.
My equations predict that isoglosses tend to get pushed away from cities, and drawn towards parts of the coast which are indented, like bays or river mouths. The city effect can be explained by imagining you live near an isogloss at the edge of a city. Because there are a lot more people on the city side of the isogloss, you will tend to have more conversations with them than with rural people living on the other side. For this reason, you will probably start using the linguistic variable used in the city. If lots of people do this, then the isogloss will move further out into the countryside.
…
My one simple assumption – that people pick up local ways of speaking – leading to equations which describe the physics of bubbles, allowed me to gain new insight into the formation of language patterns. Who knows what other linguistic patterns mathematics could explain?
…
Burridge’s paper can be found here,
Spatial Evolution of Human Dialects by James Burridge. Phys. Rev. X 7, 031008 Vol. 7, Iss. 3 — July – September 2017 Published 17 July 2017
This paper is open access and it is quite readable as these things go. In other words, you may not understand all of the mathematics, physics, or linguistics but it is written so that a relatively well informed person should be able to understand the basics if not all the nuances.