This injectable bandage could be a gamechanger (as they say) if it can be taken beyond the ‘in vitro’ (i.e., petri dish) testing stage. A May 22, 2018 news item on Nanowerk makes the announcement (Note: A link has been removed),
While several products are available to quickly seal surface wounds, rapidly stopping fatal internal bleeding has proven more difficult. Now researchers from the Department of Biomedical Engineering at Texas A&M University are developing an injectable hydrogel bandage that could save lives in emergencies such as penetrating shrapnel wounds on the battlefield (Acta Biomaterialia, “Nanoengineered injectable hydrogels for wound healing application”).
A May 22, 2018 US National Institute of Biomedical Engineering and Bioengiineering news release, which originated the news item, provides more detail (Note: Links have been removed),
The researchers combined a hydrogel base (a water-swollen polymer) and nanoparticles that interact with the body’s natural blood-clotting mechanism. “The hydrogel expands to rapidly fill puncture wounds and stop blood loss,” explained Akhilesh Gaharwar, Ph.D., assistant professor and senior investigator on the work. “The surface of the nanoparticles attracts blood platelets that become activated and start the natural clotting cascade of the body.”
Enhanced clotting when the nanoparticles were added to the hydrogel was confirmed by standard laboratory blood clotting tests. Clotting time was reduced from eight minutes to six minutes when the hydrogel was introduced into the mixture. When nanoparticles were added, clotting time was significantly reduced, to less than three minutes.
In addition to the rapid clotting mechanism of the hydrogel composite, the engineers took advantage of special properties of the nanoparticle component. They found they could use the electric charge of the nanoparticles to add growth factors that efficiently adhered to the particles. “Stopping fatal bleeding rapidly was the goal of our work,” said Gaharwar. “However, we found that we could attach growth factors to the nanoparticles. This was an added bonus because the growth factors act to begin the body’s natural wound healing process—the next step needed after bleeding has stopped.”
The researchers were able to attach vascular endothelial growth factor (VEGF) to the nanoparticles. They tested the hydrogel/nanoparticle/VEGF combination in a cell culture test that mimics the wound healing process. The test uses a petri dish with a layer of endothelial cells on the surface that create a solid skin-like sheet. The sheet is then scratched down the center creating a rip or hole in the sheet that resembles a wound.
When the hydrogel containing VEGF bound to the nanoparticles was added to the damaged endothelial cell wound, the cells were induced to grow back and fill-in the scratched region—essentially mimicking the healing of a wound.
“Our laboratory experiments have verified the effectiveness of the hydrogel for initiating both blood clotting and wound healing,” said Gaharwar. “We are anxious to begin tests in animals with the hope of testing and eventual use in humans where we believe our formulation has great potential to have a significant impact on saving lives in critical situations.”
The work was funded by grant EB023454 from the National Institute of Biomedical Imaging and Bioengineering (NIBIB), and the National Science Foundation. The results were reported in the February issue of the journal Acta Biomaterialia.
The paper was published back in April 2018 and there was an April 2, 2018 Texas A&M University news release on EurekAlert making the announcement (and providing a few unique details),
A penetrating injury from shrapnel is a serious obstacle in overcoming battlefield wounds that can ultimately lead to death.Given the high mortality rates due to hemorrhaging, there is an unmet need to quickly self-administer materials that prevent fatality due to excessive blood loss.
With a gelling agent commonly used in preparing pastries, researchers from the Inspired Nanomaterials and Tissue Engineering Laboratory have successfully fabricated an injectable bandage to stop bleeding and promote wound healing.
In a recent article “Nanoengineered Injectable Hydrogels for Wound Healing Application” published in Acta Biomaterialia, Dr. Akhilesh K. Gaharwar, assistant professor in the Department of Biomedical Engineering at Texas A&M University, uses kappa-carrageenan and nanosilicates to form injectable hydrogels to promote hemostasis (the process to stop bleeding) and facilitate wound healing via a controlled release of therapeutics.
“Injectable hydrogels are promising materials for achieving hemostasis in case of internal injuries and bleeding, as these biomaterials can be introduced into a wound site using minimally invasive approaches,” said Gaharwar. “An ideal injectable bandage should solidify after injection in the wound area and promote a natural clotting cascade. In addition, the injectable bandage should initiate wound healing response after achieving hemostasis.”
The study uses a commonly used thickening agent known as kappa-carrageenan, obtained from seaweed, to design injectable hydrogels. Hydrogels are a 3-D water swollen polymer network, similar to Jell-O, simulating the structure of human tissues.
When kappa-carrageenan is mixed with clay-based nanoparticles, injectable gelatin is obtained. The charged characteristics of clay-based nanoparticles provide hemostatic ability to the hydrogels. Specifically, plasma protein and platelets form blood adsorption on the gel surface and trigger a blood clotting cascade.
“Interestingly, we also found that these injectable bandages can show a prolonged release of therapeutics that can be used to heal the wound” said Giriraj Lokhande, a graduate student in Gaharwar’s lab and first author of the paper. “The negative surface charge of nanoparticles enabled electrostatic interactions with therapeutics thus resulting in the slow release of therapeutics.”
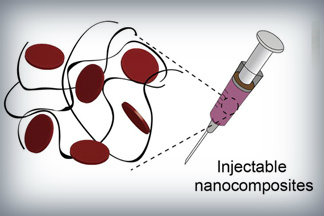
Nanoparticles that promote blood clotting and wound healing (red discs), attached to the wound-filling hydrogel component (black) form a nanocomposite hydrogel. The gel is designed to be self-administered to stop bleeding and begin wound-healing in emergency situations. Credit: Lokhande, et al. 1
Here’s a link to and a citation for the paper,
Nanoengineered injectable hydrogels for wound healing application by Giriraj Lokhande, James K. Carrow, Teena Thakur, Janet R. Xavier, Madasamy Parani, Kayla J. Bayless, Akhilesh K. Gaharwar. Acta Biomaterialia Volume 70, 1 April 2018, Pages 35-47
https://doi.org/10.1016/j.actbio.2018.01.045
This paper is behind a paywall.
Hydrogel and the brain
It’s been an interesting week for hydrogels. On May 21, 2018 there was a news item on ScienceDaily about a bioengineered hydrogel which stimulated brain tissue growth after a stroke (mouse model),
In a first-of-its-kind finding, a new stroke-healing gel helped regrow neurons and blood vessels in mice with stroke-damaged brains, UCLA researchers report in the May 21 issue of Nature Materials.
“We tested this in laboratory mice to determine if it would repair the brain in a model of stroke, and lead to recovery,” said Dr. S. Thomas Carmichael, Professor and Chair of neurology at UCLA. “This study indicated that new brain tissue can be regenerated in what was previously just an inactive brain scar after stroke.”
…
The brain has a limited capacity for recovery after stroke and other diseases. Unlike some other organs in the body, such as the liver or skin, the brain does not regenerate new connections, blood vessels or new tissue structures. Tissue that dies in the brain from stroke is absorbed, leaving a cavity, devoid of blood vessels, neurons or axons, the thin nerve fibers that project from neurons.
…
After 16 weeks, stroke cavities in mice contained regenerated brain tissue, including new neural networks — a result that had not been seen before. The mice with new neurons showed improved motor behavior, though the exact mechanism wasn’t clear.
…
Remarkable stuff.