It seems as if the first competition just ended as per my April 21, 2016 posting about the results. Ah well, out with the old and in with the ‘news’, according to an Oct. 6, 2016 news item on Nanowerk (Note: A link has been removed),
WHIZ! POW! BAM! BOOM! Today [Oct. 5, 2016], the National Science Foundation (NSF) and the National Nanotechnology Initiative (NNI) announce the opening of the second annual Generation Nano: Small Science, Superheroes! competition. The contest invites U.S. high school and home-schooled students to create a superhero that uses nanotechnology — science and technology on the scale of a nanometer, or one billionth of a meter — to solve crimes and meet today’s challenges.
By challenging students to think big (or small, in this case) to create superheroes with nanotechnology-inspired gear or powers, NSF and NNI aim to promote an early interest in science, technology, engineering and mathematics (STEM).
“An increasing number of students are drawn to the fascinating field of nanotechnology, which allows us to do things not possible before in computing, mobile communication, medicine and the environment,” said NSF Senior Advisor for Science and Engineering Mihail Roco. “The younger generation will carry on future progress in this exciting field. The Generation Nano competition gives students an opportunity to creatively combine this scientific interest with their artistic side.”
An Oct. 5, 2016 NSF news release, which originated the news item, describes the first competition and provides information for students wanting to enter this second one,
Last year’s first-ever Generation Nano competition inspired entries from more than 115 students across the U.S. The winning superhero creations included Nanoman, who battled a malignant crab-monster named Cancer; Radio Blitz, who helped dispose of local waste; and Nine, a rising superhero who used his nanosuit to defeat a pair of kidnappers.
Actor Wil Wheaton hosted the awards ceremony at the 2016 USA Science & Engineering Festival in Washington, D.C., where legendary comic book creator Stan Lee made a surprise virtual appearance to congratulate the finalists.
“The number and quality of the submissions to the Generation Nano contest last year were fantastic,” said Lisa Friedersdorf, deputy director of the National Nanotechnology Coordination Office, which provides public outreach for NNI. “I’m very excited by the four key societal missions identified for this year’s contest as nanotechnology can play a critical role in addressing each of these needs. I can’t wait to see the creative and imaginative ways the student teams take on this challenge!”
This year, participants’ superhero creations must tackle one of the following societal issues:
- Justice — Using nanotechnology to fight criminals, bullies, supervillains and other wrongdoers.
- Relief — Using nanotechnology to aid victims of famine, drought and other disasters.
- Health — Using nanotechnology to heal the sick and injured.
- Environment — Using nanotechnology to generate clean energy, control pollution and create a sustainable future.
NSF will promote the opening of the competition this week at New York Comic Con, the East Coast’s largest popular culture convention. A panel will bring together NSF-funded scientists and storytellers to talk about their imagined worlds.
Student contestants are encouraged to submit their superhero creations to the Generation Nano competition website for an opportunity to compete for prizes. A panel will review the submissions and select 15 semifinalists, and then a first and second place winner. Submissions from all semifinalists will also be posted to the Generation Nano website to allow the public to vote for their favorite superhero, which will receive a People’s Choice award.
Additional competition details
- U.S. high school and home-schooled students should submit a written entry explaining how their superhero uses nanotechnology to do good, along with a two-to-three-page comic and 90-second video.
- Competition opens Oct. 5, 2016, and submissions are due by midnight, Jan. 31, 2017 EST.
- Three rounds of judging will take place, with winners announced in the spring.
- Prizes: $1,500 for first place; $1,000 for second place; and $750 for the People’s Choice award.
Visit the Generation Nano competition website for full eligibility criteria, entry guidelines, timeline and prize information. For additional questions about the contest, contact the Generation Nano team at gennano@nsf.gov.
As noted in the news release, the competition opened Oct. 5, 2016 and entries can be submitted until Jan. 31, 2017 and you need to submit a written piece, a 2-3 page comic, and a short video.
Here’s an image from the Generation Nano website,
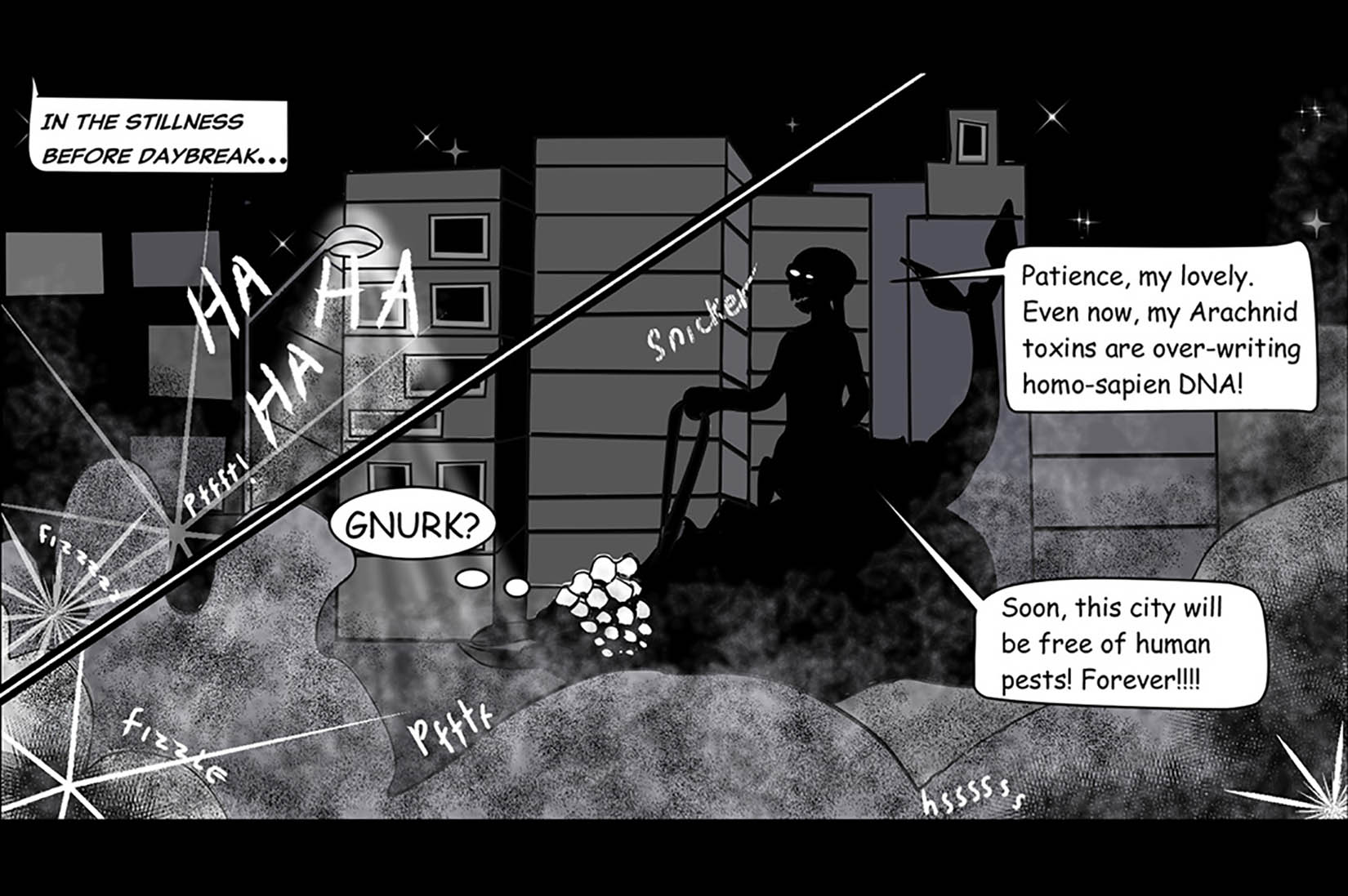 Presumably, this is from one of the entries for the first competion. You can find more images and information about the current competition here. Good luck (and remember only US-based high school and home-schooled students can submit.)
Presumably, this is from one of the entries for the first competion. You can find more images and information about the current competition here. Good luck (and remember only US-based high school and home-schooled students can submit.)