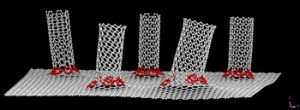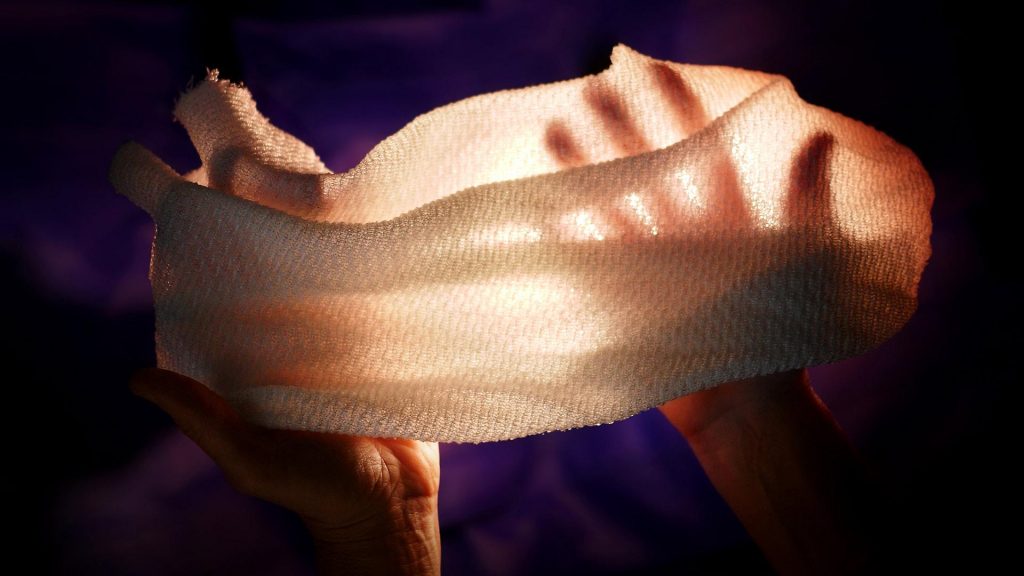
This may look like just another gauzy fabric but it has some special properties according to a February 7, 2019 news item on ScienceDaily,
Despite decades of innovation in fabrics with high-tech thermal properties that keep marathon runners cool or alpine hikers warm, there has never been a material that changes its insulating properties in response to the environment. Until now.
University of Maryland researchers have created a fabric that can automatically regulate the amount of heat that passes through it. When conditions are warm and moist, such as those near a sweating body, the fabric allows infrared radiation (heat) to pass through. When conditions become cooler and drier, the fabric reduces the heat that escapes. The development was reported in the February 8, 2019 issue of the journal Science.
…
A February 8, 2019 University of Maryland news release (also on EurekAlert [published Feb.7, 2019]) by Kimbra Cutlip delves further into the research,
The researchers created the fabric from specially engineered yarn coated with a conductive metal. Under hot, humid conditions, the strands of yarn compact and activate the coating, while cool, dry conditions reverse the action. The researchers refer to this as “gating”—essentially a tunable blind that transmits or blocks heat.
“This is the first technology that allows us to dynamically gate infrared radiation,” said YuHuang Wang, a professor of chemistry and biochemistry and one of the paper’s corresponding authors who directed the studies.
The base yarn for this new textile is created with fibers made of two different synthetic materials—one absorbs water and the other repels it. The strands are coated with carbon nanotubes, a special class of lightweight, carbon-based, conductive metal.
Because materials in the fibers both resist and absorb water, the fibers warp when exposed to humidity such as that surrounding a sweating body. That distortion brings the strands of yarn closer together, opening the pores in the fabric and creating a minor cooling effect by allowing heat to escape. More importantly, it modifies the electromagnetic coupling between the carbon nanotubes in the coating.
“You can think of this coupling effect like the bending of a radio antenna to change the wavelength or frequency it resonates with,” Wang said. “Imagine bringing two antennae close together to regulate the kind of electromagnetic wave they pick up. When the fabric fibers are brought closer together, the radiation they interact with changes. In clothing, that means the fabric interacts with the heat radiating from the human body.
”Depending on the tuning, the fabric either blocks infrared radiation or allows it to pass through. The reaction is almost instant, so before people realize it, the dynamic gating mechanism is either cooling them down or working in reverse to trap heat.
“The human body is a perfect radiator. It gives off heat quickly,” said Min Ouyang, a professor of physics at UMD and the paper’s other corresponding author. “For all of history, the only way to regulate the radiator has been to take clothes off or put clothes on. But this fabric is a true bidirectional regulator.More work is needed before the fabric can be commercialized, but according to the researchers, materials used for the base fiber are readily available and the carbon coating can be easily added during the standard dyeing process.
Here’s a link to and a citation for the paper,
Dynamic gating of infrared radiation in a textile by Xu A. Zhang, Shangjie Yu, Beibei Xu, Min Li, Zhiwei Peng, Yongxin Wang, Shunliu Deng, Xiaojian Wu, Zupeng Wu, Min Ouyang, YuHuang Wang. Science 08 Feb 2019: Vol. 363, Issue 6427, pp. 619-623 DOI: 10.1126/science.aau1217
This paper is behind a paywall.