I have two stories about lungs and they are entirely different with the older one being a bioengineering story from the US and the more recent one being an artificial tissue story from the University of Toronto and the University of Ottawa (both in Canada).
Lab grown lungs
The Canadian Broadcasting Corporation’s Quirks and Quarks radio programme posted a December 29, 2018 news item (with embedded radio files) about bioengineered lunjgs,
There are two major components to building an organ: the structure and the right cells on that structure. A team led by Dr. Joan Nichols, a Professor of Internal Medicine, Microbiology and Immunology at the University of Texas Medical Branch in Galveston, were able to tackle both parts of the problem
In their experiment they used a donor organ for the structure. They took a lung from an unrelated pig, and stripped it of its cells, leaving a scaffold of collagen, a tough, flexible protein. This provided a pre-made appropriate structure, though in future they think it may be possible to use 3-D printing technology to get the same result.They then added cultured cells from the animal who would be receiving the transplant – so the lung was made of the animal’s own cells. Cultured lung and blood vessel cells were placed on the scaffold and it was placed in a tank for 30 days with a cocktail of nutrients to help the cells stick to the scaffold and proliferate. The result was a kind of baby lung.
They then transplanted the bio-engineered, though immature, lung into the recipient animal where they hoped it would continue to develop and mature – growing to become a healthy, functioning organ.
The recipients of the bio-engineered lungs were four pigs adult pigs, which appeared to tolerate the transplants well. In order to study the development of the bio-engineered lungs, they euthanized the animals at different times: 10 hours, two weeks, one month and two months after transplantation.
They found that as early as two weeks, the bio-engineered lung had integrated into the recipient animals’ body, building a strong network of blood vessels essential for the lung to survive. There was no evidence of pulmonary edema, the build of fluid in the lungs, which is usually a sign of the blood vessels not working efficiently. There was no sign of rejection of the transplanted organs, and the pigs were healthy up to the point where they were euthanized.
One lingering concern is how well the bio-engineered lungs delivered oxygen. The four pigs who received the trasplant [sic] had one original functioning lung, so they didn’t depend on their new bio-engineered lung for breathing. The scientists were not sure that the bio-engineered lung was mature enough to handle the full load of oxygen on its own.
…
You can hear Bob McDonald’s (host of Quirks & Quarks, a Canadian Broadcasting Corporation science radio programme) interview lead scientist, Dr. Joan Nichols if you go to here. (Note: I find he overmodulates his voice but some may find he has a ‘friendly’ voice.)
This is an image of the lung scaffold produced by the team,
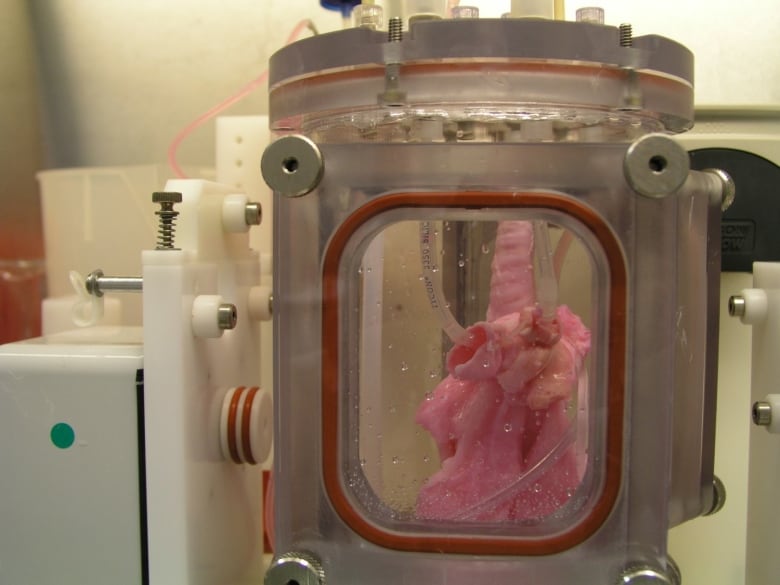
Here’s more technical detail in an August 1, 2018i University of Texas Medical Branch (UTMB) news release (also on EurekAlert), which originally announced the research,
A research team at the University of Texas Medical Branch at Galveston have bioengineered lungs and transplanted them into adult pigs with no medical complication.
In 2014, Joan Nichols and Joaquin Cortiella from The University of Texas Medical Branch at Galveston were the first research team to successfully bioengineer human lungs in a lab. In a paper now available in Science Translational Medicine, they provide details of how their work has progressed from 2014 to the point no complications have occurred in the pigs as part of standard preclinical testing.
“The number of people who have developed severe lung injuries has increased worldwide, while the number of available transplantable organs have decreased,” said Cortiella, professor of pediatric anesthesia. “Our ultimate goal is to eventually provide new options for the many people awaiting a transplant,” said Nichols, professor of internal medicine and associate director of the Galveston National Laboratory at UTMB.
To produce a bioengineered lung, a support scaffold is needed that meets the structural needs of a lung. A support scaffold was created using a lung from an unrelated animal that was treated using a special mixture of sugar and detergent to eliminate all cells and blood in the lung, leaving only the scaffolding proteins or skeleton of the lung behind. This is a lung-shaped scaffold made totally from lung proteins.
The cells used to produce each bioengineered lung came from a single lung removed from each of the study animals. This was the source of the cells used to produce a tissue-matched bioengineered lung for each animal in the study. The lung scaffold was placed into a tank filled with a carefully blended cocktail of nutrients and the animals’ own cells were added to the scaffold following a carefully designed protocol or recipe. The bioengineered lungs were grown in a bioreactor for 30 days prior to transplantation. Animal recipients were survived for 10 hours, two weeks, one month and two months after transplantation, allowing the research team to examine development of the lung tissue following transplantation and how the bioengineered lung would integrate with the body.
All of the pigs that received a bioengineered lung stayed healthy. As early as two weeks post-transplant, the bioengineered lung had established the strong network of blood vessels needed for the lung to survive.
“We saw no signs of pulmonary edema, which is usually a sign of the vasculature not being mature enough,” said Nichols and Cortiella. “The bioengineered lungs continued to develop post-transplant without any infusions of growth factors, the body provided all of the building blocks that the new lungs needed.”
Nichols said that the focus of the study was to learn how well the bioengineered lung adapted and continued to mature within a large, living body. They didn’t evaluate how much the bioengineered lung provided oxygenation to the animal.
“We do know that the animals had 100 percent oxygen saturation, as they had one normal functioning lung,” said Cortiella. “Even after two months, the bioengineered lung was not yet mature enough for us to stop the animal from breathing on the normal lung and switch to just the bioengineered lung.”
For this reason, future studies will look at long-term survival and maturation of the tissues as well as gas exchange capability.
The researchers said that with enough funding, they could grow lungs to transplant into people in compassionate use circumstances within five to 10 years.
“It has taken a lot of heart and 15 years of research to get us this far, our team has done something incredible with a ridiculously small budget and an amazingly dedicated group of people,” Nichols and Cortiella said.
Here’s a citation and another link for the paper,
Production and transplantation of bioengineered lung into a large-animal model by Joan E. Nichols, Saverio La Francesca, Jean A. Niles, Stephanie P. Vega, Lissenya B. Argueta, Luba Frank, David C. Christiani, Richard B. Pyles, Blanca E. Himes, Ruyang Zhang, Su Li, Jason Sakamoto, Jessica Rhudy, Greg Hendricks, Filippo Begarani, Xuewu Liu, Igor Patrikeev, Rahul Pal, Emiliya Usheva, Grace Vargas, Aaron Miller, Lee Woodson, Adam Wacher, Maria Grimaldo, Daniil Weaver, Ron Mlcak, and Joaquin Cortiella. Science Translational Medicine 01 Aug 2018: Vol. 10, Issue 452, eaao3926 DOI: 10.1126/scitranslmed.aao3926
This paper is behind a paywall.
Artificial lung cancer tissue
The research teams at the University of Toronto and the University of Ottawa worked on creating artificial lung tissue but other applications are possible too. First, there’s the announcement in a February 25, 2019 news item on phys.org,
A 3-D hydrogel created by researchers in U of T Engineering Professor Molly Shoichet’s lab is helping University of Ottawa researchers to quickly screen hundreds of potential drugs for their ability to fight highly invasive cancers.
Cell invasion is a critical hallmark of metastatic cancers, such as certain types of lung and brain cancer. Fighting these cancers requires therapies that can both kill cancer cells as well as prevent cell invasion of healthy tissue. Today, most cancer drugs are only screened for their ability to kill cancer cells.
“In highly invasive diseases, there is a crucial need to screen for both of these functions,” says Shoichet. “We now have a way to do this.”
A February 25, 2019 University of Toronto news release (also on EurekAlert), which originated the news item, offers more detail ,
In their latest research, the team used hydrogels to mimic the environment of lung cancer, selectively allowing cancer cells, and not healthy cells, to invade. In their latest research, the team used hydrogels to mimic the environment of lung cancer, selectively allowing cancer cells, and not healthy cells, to invade. This emulated environment enabled their collaborators in Professor Bill Stanford’s lab at University of Ottawa to screen for both cancer-cell growth and invasion. The study, led by Roger Y. Tam, a research associate in Shochet’s lab, was recently published in Advanced Materials.
“We can conduct this in a 384-well plate, which is no bigger than your hand. And with image-analysis software, we can automate this method to enable quick, targeted screenings for hundreds of potential cancer treatments,” says Shoichet.
One example is the researchers’ drug screening for lymphangioleiomyomatosis (LAM), a rare lung disease affecting women. Shoichet and her team were inspired by the work of Green Eggs and LAM, a Toronto-based organization raising awareness of the disease.
Using their hydrogels, they were able to automate and screen more than 800 drugs, thereby uncovering treatments that could target disease growth and invasion.
In the ongoing collaboration, the researchers plan to next screen multiple drugs at different doses to gain greater insight into new treatment methods for LAM. The strategies and insights they gain could also help identify new drugs for other invasive cancers.
Shoichet, who was recently named a Distinguished Woman in Chemistry or Chemical Engineering, also plans to patent the hydrogel technology.
“This has, and continues to be, a great collaboration that is advancing knowledge at the intersection of engineering and biology,” says Shoichet.
I note that Shoichet (pronounced ShoyKet) is getting ready to patent this work. I do have a question about this and it’s not up to Shoichet to answer as she didn’t create the system. Will the taxpayers who funded her work receive any financial benefits should the hydrogel prove to be successful or will we be paying double, both supporting her research and paying for the hydrogel through our healthcare costs?
Getting back to the research, here’s a link to and a citation for the paper,
Rationally Designed 3D Hydrogels Model Invasive Lung Diseases Enabling High‐Content Drug Screening by Roger Y. Tam, Julien Yockell‐Lelièvre, Laura J. Smith, Lisa M. Julian, Alexander E. G. Baker, Chandarong Choey, Mohamed S. Hasim, Jim Dimitroulakos, William L. Stanford, Molly S. Shoichet. Advanced Materials Volume 31, Issue 7 February 15, 2019 1806214 First published online: 27 December 2018 DOI: https://doi.org/10.1002/adma.201806214
This paper is behind a paywall.