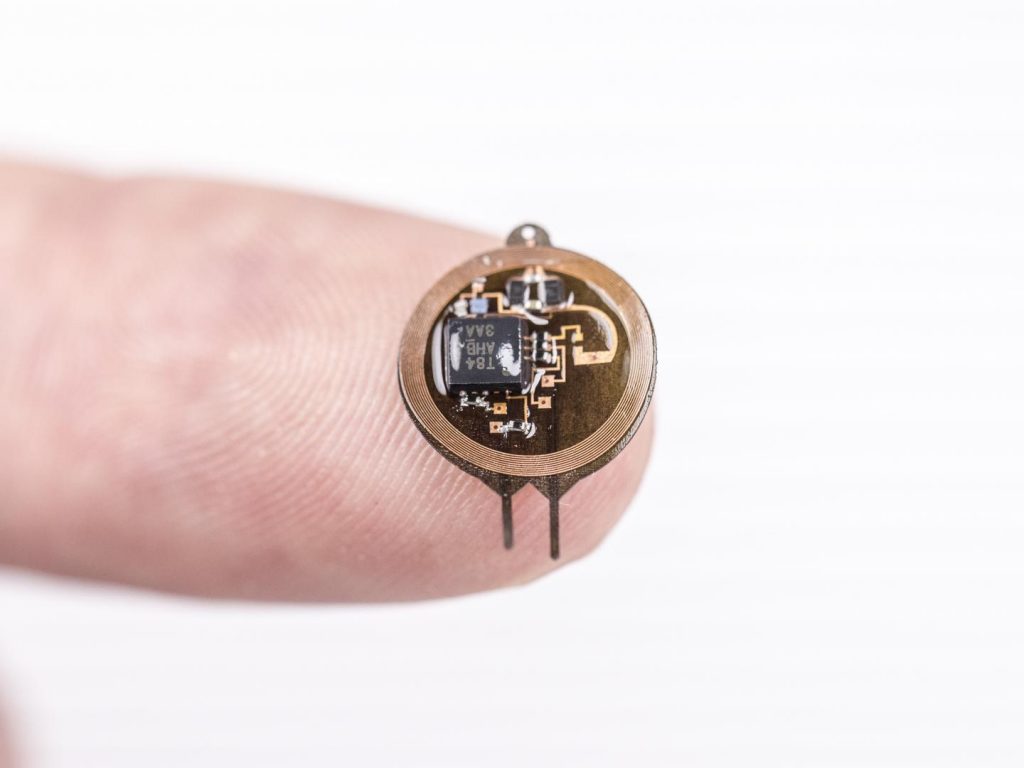
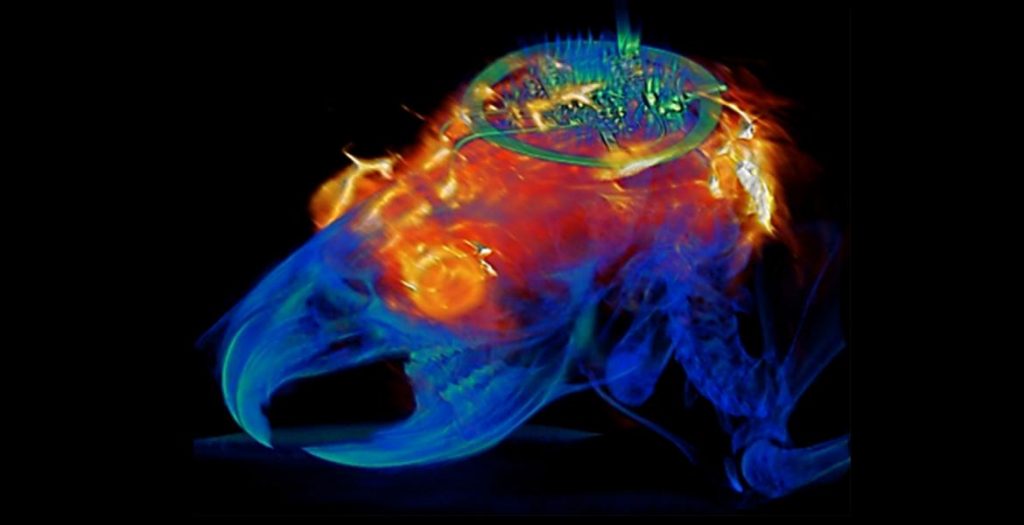
A March 19, 2021 Northwestern University news release on EurekAlert announces the creation of a device designed to monitor brain organoids (for anyone unfamiliar with brain organoids there’s more information after the news),
A team of scientists, led by researchers at Northwestern University, Shirley Ryan AbilityLab and the University of Illinois at Chicago (UIC), has developed novel technology promising to increase understanding of how brains develop, and offer answers on repairing brains in the wake of neurotrauma and neurodegenerative diseases.
Their research is the first to combine the most sophisticated 3-D bioelectronic systems with highly advanced 3-D human neural cultures. The goal is to enable precise studies of how human brain circuits develop and repair themselves in vitro. The study is the cover story for the March 19 [March 17, 2021 according to the citation] issue of Science Advances.
The cortical spheroids used in the study, akin to “mini-brains,” were derived from human-induced pluripotent stem cells. Leveraging a 3-D neural interface system that the team developed, scientists were able to create a “mini laboratory in a dish” specifically tailored to study the mini-brains and collect different types of data simultaneously. Scientists incorporated electrodes to record electrical activity. They added tiny heating elements to either keep the brain cultures warm or, in some cases, intentionally overheated the cultures to stress them. They also incorporated tiny probes — such as oxygen sensors and small LED lights — to perform optogenetic experiments. For instance, they introduced genes into the cells that allowed them to control the neural activity using different-colored light pulses.
This platform then enabled scientists to perform complex studies of human tissue without directly involving humans or performing invasive testing. In theory, any person could donate a limited number of their cells (e.g., blood sample, skin biopsy). Scientists can then reprogram these cells to produce a tiny brain spheroid that shares the person’s genetic identity. The authors believe that, by combining this technology with a personalized medicine approach using human stem cell-derived brain cultures, they will be able to glean insights faster and generate better, novel interventions.
“The advances spurred by this research will offer a new frontier in the way we study and understand the brain,” said Shirley Ryan AbilityLab’s Dr. Colin Franz, co-lead author on the paper who led the testing of the cortical spheroids. “Now that the 3-D platform has been developed and validated, we will be able to perform more targeted studies on our patients recovering from neurological injury or battling a neurodegenerative disease.”
Yoonseok Park, postdoctoral fellow at Northwestern University and co-lead author, added, “This is just the beginning of an entirely new class of miniaturized, 3-D bioelectronic systems that we can construct to expand the capacity of the regenerative medicine field. For example, our next generation of device will support the formation of even more complex neural circuits from brain to muscle, and increasingly dynamic tissues like a beating heart.”
Current electrode arrays for tissue cultures are 2-D, flat and unable to match the complex structural designs found throughout nature, such as those found in the human brain. Moreover, even when a system is 3-D, it is extremely challenging to incorporate more than one type of material into a small 3-D structure. With this advance, however, an entire class of 3-D bioelectronics devices has been tailored for the field of regenerative medicine.
“Now, with our small, soft 3-D electronics, the capacity to build devices that mimic the complex biological shapes found in the human body is finally possible, providing a much more holistic understanding of a culture,” said Northwestern’s John Rogers, who led the technology development using technology similar to that found in phones and computers. “We no longer have to compromise function to achieve the optimal form for interfacing with our biology.”
As a next step, scientists will use the devices to better understand neurological disease, test drugs and therapies that have clinical potential, and compare different patient-derived cell models. This understanding will then enable a better grasp of individual differences that may account for the wide variation of outcomes seen in neurological rehabilitation.
“As scientists, our goal is to make laboratory research as clinically relevant as possible,” said Kristen Cotton, research assistant in Dr. Franz’s lab. “This 3-D platform opens the door to new experiments, discovery and scientific advances in regenerative neurorehabilitation medicine that have never been possible.”
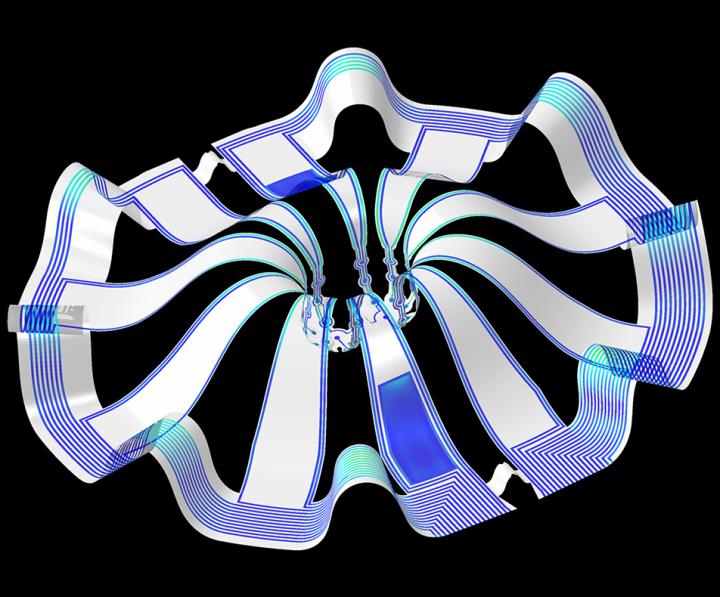
As for what brain ogranoids might be, Carl Zimmer in an Aug. 29, 2019 article for the New York Times provides an explanation,
Organoids Are Not Brains. How Are They Making Brain Waves?
Two hundred and fifty miles over Alysson Muotri’s head, a thousand tiny spheres of brain cells were sailing through space.
The clusters, called brain organoids, had been grown a few weeks earlier in the biologist’s lab here at the University of California, San Diego. He and his colleagues altered human skin cells into stem cells, then coaxed them to develop as brain cells do in an embryo.
The organoids grew into balls about the size of a pinhead, each containing hundreds of thousands of cells in a variety of types, each type producing the same chemicals and electrical signals as those cells do in our own brains.
In July, NASA packed the organoids aboard a rocket and sent them to the International Space Station to see how they develop in zero gravity.
Now the organoids were stowed inside a metal box, fed by bags of nutritious broth. “I think they are replicating like crazy at this stage, and so we’re going to have bigger organoids,” Dr. Muotri said in a recent interview in his office overlooking the Pacific.
What, exactly, are they growing into? That’s a question that has scientists and philosophers alike scratching their heads.
On Thursday, Dr. Muotri and his colleagues reported that they have recorded simple brain waves in these organoids. In mature human brains, such waves are produced by widespread networks of neurons firing in synchrony. Particular wave patterns are linked to particular forms of brain activity, like retrieving memories and dreaming.
As the organoids mature, the researchers also found, the waves change in ways that resemble the changes in the developing brains of premature babies.
“It’s pretty amazing,” said Giorgia Quadrato, a neurobiologist at the University of Southern California who was not involved in the new study. “No one really knew if that was possible.”
But Dr. Quadrato stressed it was important not to read too much into the parallels. What she, Dr. Muotri and other brain organoid experts build are clusters of replicating brain cells, not actual brains.
…
If you have the time, I recommend reading Zimmer’s article in its entirety. Perhaps not coincidentally, Zimmer has an excerpt titled “Lab-Grown Brain Organoids Aren’t Alive. But They’re Not Not Alive, Either.” published in Slate.com,
From Life’s Edge: The Search For What It Means To Be Alive by Carl Zimmer, published by Dutton, an imprint of Penguin Publishing Group, a division of Penguin Random House, LLC. Copyright © 2021 by Carl Zimmer.
Cleber Trujillo led me to a windowless room banked with refrigerators, incubators, and microscopes. He extended his blue-gloved hands to either side and nearly touched the walls. “This is where we spend half our day,” he said.
In that room Trujillo and a team of graduate students raised a special kind of life. He opened an incubator and picked out a clear plastic box. Raising it above his head, he had me look up at it through its base. Inside the box were six circular wells, each the width of a cookie and filled with what looked like watered-down grape juice. In each well 100 pale globes floated, each the size of a housefly head.
…
Getting back to the research about monitoring brain organoids, here’s a link to and a citation for the paper about cortical spheroids,
Three-dimensional, multifunctional neural interfaces for cortical spheroids and engineered assembloids by Yoonseok Park, Colin K. Franz, Hanjun Ryu, Haiwen Luan, Kristen Y. Cotton, Jong Uk Kim, Ted S. Chung, Shiwei Zhao, Abraham Vazquez-Guardado, Da Som Yang, Kan Li, Raudel Avila, Jack K. Phillips, Maria J. Quezada, Hokyung Jang, Sung Soo Kwak, Sang Min Won, Kyeongha Kwon, Hyoyoung Jeong, Amay J. Bandodkar, Mengdi Han, Hangbo Zhao, Gabrielle R. Osher, Heling Wang, KunHyuck Lee, Yihui Zhang, Yonggang Huang, John D. Finan and John A. Rogers. Science Advances 17 Mar 2021: Vol. 7, no. 12, eabf9153 DOI: 10.1126/sciadv.abf9153
This paper appears to be open access.
According to a March 22, 2021 posting on the Shirley Riley AbilityLab website, the paper is featured on the front cover of Science Advances (vol. 7 no. 12).