Mille-feuille is a pastry and it’s name translates to ‘a thousand leaves’, which hints at how a ‘mille-feuille’ nanofilter is constructed. From a May 18, 2016 news item on Nanowerk,
A simple paper sheet made by scientists at Uppsala University can improve the quality of life for millions of people by removing resistant viruses from water. The sheet, made of cellulose nanofibers, is called the mille-feuille filter as it has a unique layered internal architecture resembling that of the French puff pastry mille-feuille (Eng. thousand leaves).
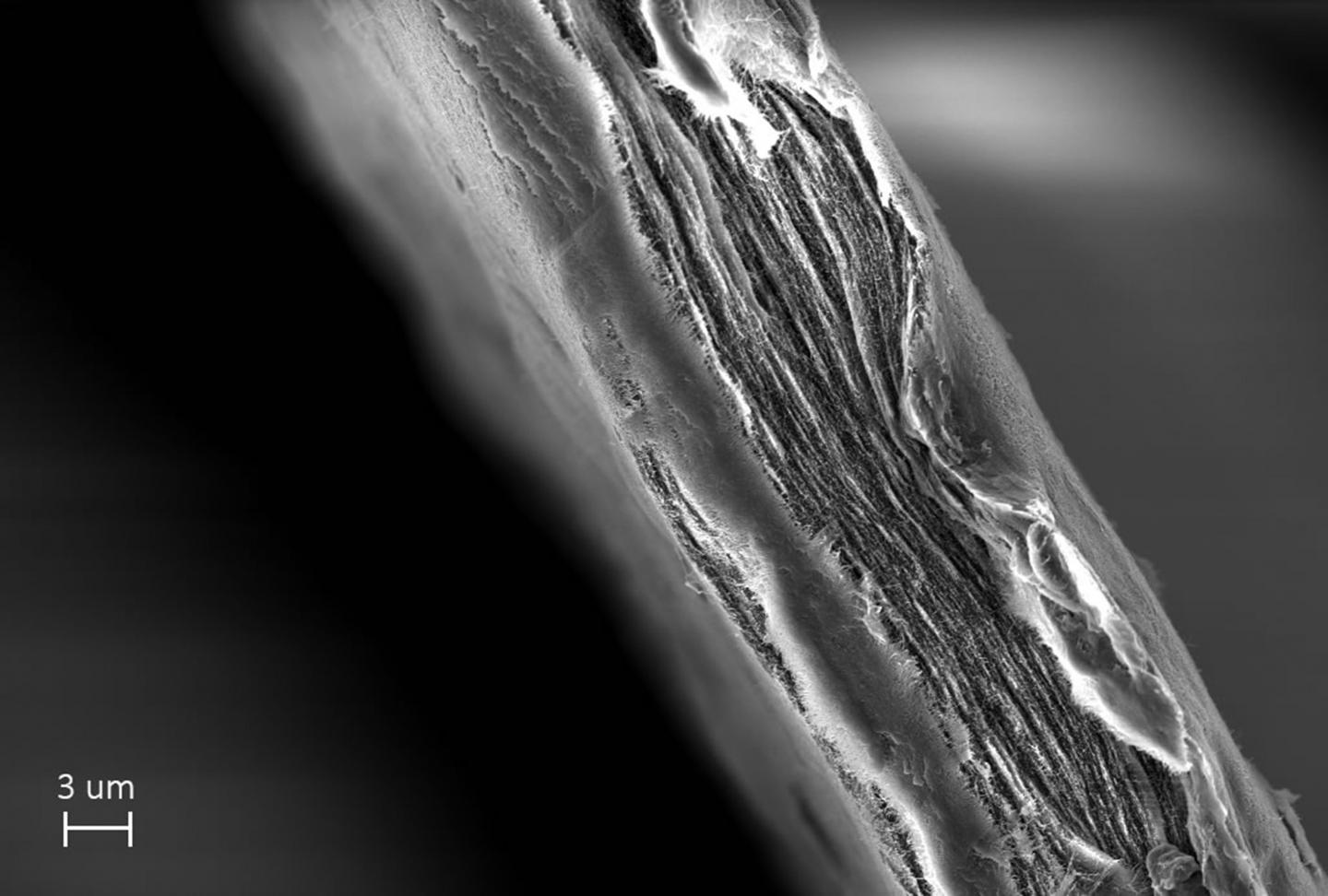
Caption: The sheet made of cellulose nanofibers in the mille-feuille filter which can remove resistant viruses from water. Research led by Albert Mihranyan, Professor of Nanotechnology at Uppsala University, Image by Simon Gustafsson. Credit: Simon Gustafsson
A May 18, 2016 Uppsala University (Sweden) press release on EurekAlert, which originated the news item, expands on the theme,
With a filter material directly from nature, and by using simple production methods, we believe that our filter paper can become the affordable global water filtration solution and help save lives. Our goal is to develop a filter paper that can remove even the toughest viruses from water as easily as brewing coffee’, says Albert Mihranyan, Professor of Nanotechnology at Uppsala University, who heads the study.
Access to safe drinking water is among the UN’s Sustainable Development Goals. More than 748 million people lack access to safe drinking water and basic sanitation. Water-borne infections are among the global causes for mortality, especially in children under age of five, and viruses are among the most notorious water-borne infectious microorganisms. They can be both extremely resistant to disinfection and difficult to remove by filtration due to their small size.
Today we heavily rely on chemical disinfectants, such as chlorine, which may produce toxic by-products depending on water quality. Filtration is a very effective, robust, energy-efficient, and inert method of producing drinking water as it physically removes the microorganisms from water rather than inactivates them. But the high price of efficient filters is limiting their use today.
‘Safe drinking water is a problem not only in the low-income countries. Massive viral outbreaks have also occurred in Europe in the past, including Sweden, continues Mihranyan referring to the massive viral outbreak in Lilla Edet municipality in Sweden in 2008, when more than 2400 people or almost 20% of the local population got infected with Norovirus due to poor water. ‘ Cellulose is one of the most common filtering media used in daily life from tea-bags to vacuum cleaners. However, the general-purpose filter paper has too large pores to remove viruses. In 2014, the group has described for the first time a paper filter that can remove large size viruses, such as influenza virus.
Small size viruses have been much harder to get rid of, as they are extremely resistant to physical and chemical inactivation. A successful filter should not only remove viruses but also feature high flow, low fouling, and long life-time, which makes advanced filters very expensive to develop. Now, with the breakthrough achieved using the mille-feuille filter the long awaited shift to affordable advanced filtration solutions may at last become a reality. Another application of the filter includes production of therapeutic proteins and vaccines.
Here’s a link to and a citation for the paper,
Mille-feuille paper: a novel type of filter architecture for advanced virus separation applications by Simon Gustafsson, Pascal Lordat, Tobias Hanrieder, Marcel Asper, Oliver Schaeferb, and Albert Mihranyan, Mater. Horiz., 2016, Advance Article DOI: 10.1039/C6MH00090H First published online 18 May 2016
This paper is behind a paywall.