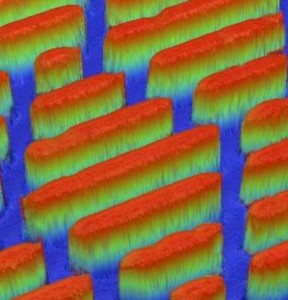
In the past several years, scientists have created antibacterial surfaces by fabricating materials with specific types of nanostructures. According to a May 27, 2020 news item on Nanowerk, scientists have now been able to add antiviral properties (Note: A link has been removed),
The novel coronavirus pandemic has caused an increased demand for antimicrobial treatments that can keep surfaces clean, particularly in health care settings. Although some surfaces have been developed that can combat bacteria, what’s been lacking is a surface that can also kill off viruses.
Now, researchers have found a way to impart durable antiviral and antibacterial properties to an aluminum alloy used in hospitals, according to a report in ACS Biomaterials Science & Engineering (“Antiviral and Antibacterial Nanostructured Surfaces with Excellent Mechanical Properties for Hospital Applications”).
…
A May 27, 2020 American Chemical Society (ACS) news release (also on EurekAlert), which originated the news item, describes the problem and the proposed solution,
Among other mechanisms, viruses and bacteria can spread when a person touches a site where germs have settled, such as a doorframe, handrail or medical device. A healthy person can often fight off these bugs, but hospital patients can be more vulnerable to infection. The number of hospital-acquired infections has been on the decline in the U.S., but they still cause tens of thousands of deaths every year, according to the U.S. Department of Health and Human Services. Chemical disinfectants or coatings containing hydrophobic compounds, silver ions or copper can reduce infectious contaminants on surfaces, but these treatments don’t last. However, nature has developed its own solutions for battling microorganisms, including microscopic structural features that render some insect wings lethal to bacteria. Scientists have replicated this effect by forming surfaces covered with minute pillars and other shapes that distort and kill bacterial cells. But Prasad Yarlagadda and colleagues wanted to inactivate viruses as well as bacteria, so they set out to generate a novel nanoscale topography on long-lasting, industrially relevant materials.
The team experimented with disks of aluminum 6063, which is used in doorframes, window panels, and hospital and medical equipment. Etching the disks with sodium hydroxide for up to 3 hours changed the initially smooth, hydrophobic surface into a ridged, hydrophilic surface. Bacteria or viruses were then applied to the etched disks. Most of the Pseudomonas aeruginosa and Staphylococcus aureus bacteria were inactivated after 3 hours on the surface, while viability of common respiratory viruses dropped within 2 hours; both results were better than with plastic or smooth aluminum surfaces. The disks retained their effectiveness even after tests designed to mimic hospital wear and tear. The researchers note this is the first report to show combined antibacterial and antiviral properties of a durable, nanostructured surface that has the potential to stop the spread of infections arising from physical surfaces in hospitals. This strategy could be extended to surfaces in other public areas, such as cruise ships, planes and airports, they say. The team is now studying the effects of their nano-textured aluminum surfaces on the novel coronavirus.
This approach reminds me of Sharklet, a company fabricating a material designed to mimic a shark’s skin which is naturally antibacterial due to the nanostructures on its skin (see my September 18, 2014 posting).
More about Sharklet later. First, here’s a link to and a citation for the paper about this latest work,
Antiviral and Antibacterial Nanostructured Surfaces with Excellent Mechanical Properties for Hospital Applications by Jafar Hasan, Yanan Xu, Tejasri Yarlagadda, Michael Schuetz, Kirsten Spann, and Prasad KDV Yarlagadda. ACS Biomater. Sci. Eng. 2020, XXXX, XXX, XXX-XXX DOI: https://doi.org/10.1021/acsbiomaterials.0c00348 Publication Date:May 7, 2020 Copyright © 2020 American Chemical Society
This paper is behind a paywall.
Business and science: a Sharklet update
You can find the Sharklet website here. I wasn’t able to find any news about recent business deals other than the company’s acquisition by Peaceful Union in May 2017. From a May 17, 2017 Sharklet news release on Business Wire (and on the company website here),
Sharklet Technologies, Inc., a biotechnology company lauded for the creation and commercialization of Sharklet®, the world’s first micro-texture that inhibits bacterial growth on surfaces, has announced that it has completed a financing event led by Peaceful Union, an equity medical device firm in Hangzhou, China. Terms of the transaction were not disclosed.
The acquisition of the company will enable Sharklet Technologies to accelerate the development of Sharklet for medical devices where chemical-free bacterial inhibition is desired as well as high-touch surfaces prone to bacterial contamination. The company also will accelerate development of a newly enhanced wound dressing technology to encourage healing.
Joe Bagan and Mark Spiecker led the transaction structure. “This is an important day for the company and investors,” said Joe Bagan, former board chair, and Mark Spiecker, former CEO. “Our investors will realize a significant transaction while enabling the company to accelerate growth.”
In concert with the investment, Sharklet Technologies founding member, chief technology officer, and Sharklet inventor Dr. Anthony Brennan, will become chairman of the board assuming duties from chairman Joe Bagan and CEO Mark Spiecker.
…
Interestingly, Bagan and Spiecker are Chief Executive Officer (CEO) and President, respectively at STAQ Pharma. I wonder if there are plans to sell this company too.
Getting back to Sharklet, I found two items of recent origin about business but I cannot speak to the accuracy or trustworthiness of either item. That said, you will find they provide some detail about Sharklet’s new business directions and new business ties.
While Sharklet’s current business associations have a sketchy quality, it seems that’s not unusual in business, especially where new technologies are concerned. For example, the introduction of electricity into homes and businesses was a tumultuous affair as the 2008 book, ‘Power Struggles; Scientific Authority and the Creation of Practical Electricity Before Edison’ by Michael Brian Schiffer makes clear, from the MIT [Massachusetts Institute of Technology] Press ‘Power Struggles’ webpage,
In 1882, Thomas Edison and his Edison Electric Light Company unveiled the first large-scale electrical system in the world to light a stretch of offices in a city. … After laying out a unified theoretical framework for understanding technological change, Schiffer presents a series of fascinating case studies of pre-Edison electrical technologies, including Volta’s electrochemical battery, the blacksmith’s electric motor, the first mechanical generators, Morse’s telegraph, the Atlantic cable, and the lighting of the Capitol dome. Schiffer discusses claims of “practicality” and “impracticality” (sometimes hotly contested) made for these technologies, and examines the central role of the scientific authority—in particular, the activities of Joseph Henry, mid-nineteenth-century America’s foremost scientist—in determining the fate of particular technologies. These emerging electrical technologies formed the foundation of the modern industrial world. Schiffer shows how and why they became commercial products in the context of an evolving corporate capitalism in which conflicting judgments of practicality sometimes turned into power struggles. [emphases mine]
Even given that the book’s focus is pre-Edison electricity, how do you mention Edison himself without even casually mentioning Nikola Tesla and George Westinghouse in the book’s overview? Getting back to my point, emerging technologies do not emerge easily.