It was a bit of a stretch to include Diana Ross in a Jan. 12, 2015 news item on Nanowerk about nanophotonic research at the University of Twente’s MESA+ Institute for Nanotechnology but I’m glad they did,
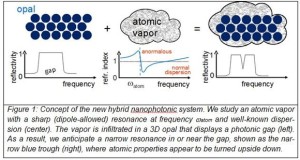
Ever since the early 1900s work of Niels Bohr and Hendrik Lorentz, it is known that atoms display characteristic resonant behavior to light. The hallmark of a resonance is its characteristic peak-trough behavior of the refractive index with optical frequency. Scientists from the Dutch MESA+ Institute for Nanotechnology at the University of Twente have recently infiltrated cesium atoms in a self-assembled opal to create a hybrid nanophotonic system. By tuning the opal’s forbidden gap relative to the atomic resonance, dramatic changes are observed in reflectivity. In the most extreme case, the atomic reflection spectrum is turned upside down[1] compared to the traditional case. Since dispersion is crucial in the control of optical signal pulses, the new results offer opportunities for optical information manipulation. As atoms are exquisite storage devices for light quanta, the results open vistas on quantum information processing, as well as on new nanoplasmonics.
A Jan. 12, 2015 MESA+ Institute for Nanotechnology at the University of Twente press release, which originated the news item, provides an illustrative diagram and a wealth of technical detail about the research,
While the speed of light c is proverbial, it can readily be modified by sending light through a medium with a certain refractive index n. In the medium, the speed will be decreased by the index to c/n. In any material, the refractive index depends on the frequency of the light. Usually the refractive index increases with frequency, called normal dispersion as it prevails at most frequencies in most materials such as a glass of water, a telecom fiber, or an atomic vapor. Close to the resonance frequency of the material, the index strongly decreases, called anomalous dispersion.
Dispersion is essential to control how optical bits of information – encoded as short pulses – is manipulated optical circuits. In modern optics at the nanoscale, called nanophotonics, dispersion is controlled with classes of complex nanostructures that cause novel behavior to emerge. An example is a photonic crystal fiber, which does not consist of only glass like a traditional fiber, but of an intricate arrangement of holes and glass nanostructures.
The Twente team led by Harding devised a hybrid system consisting of an atomic vapor infiltrated in an opal photonic crystal. Photonic crystals have attracted considerable attention for their ability to radically control propagation and emission of light. These nanostructures are well-known for their ability to control the emission and propagation of light. The opals have a periodic variation of the refractive index (see Figure 1) that ensures that a certain color of light is forbidden to exist inside the opal. The light cannot enter the opal as it is reflected, which is called a gap (see Figure 1). In an analogy to semiconductors, such an effect is called a “photonic band gap”. Photonic gaps are at the basis of tiny on-chip light sources and lasers, efficient solar cells, invisibility cloaks, and devices to process optical information.
The Twente team changed the index of refraction of the voids in a photonic crystal by substituting the air by a vapor of atoms with a strong resonance, as shown in Figure 1. The contrast of the refractive index between the vapor and the opal’s silica nanospheres was effectively used as a probe. The density of the cesium vapor was greatly varied by changing the temperature in the cell up to 420 K. At the same time, the photonic gap of the opal shifted relative to the atomic resonance due to a slow chemical reaction between the opal’s backbone material (silica) and the cesium.
On resonance, light excites an atom to a higher state and subsequently the atom reemits the light. Hence, an atom behaves like a little cavity that stores light. Simultaneously the index of refraction changes strongly for colors near resonance. For slightly longer wavelengths the index of refraction is high, on resonance it is close to one, and slightly shorter wavelengths it can even decrease below one. This effect of the cesium atoms is clearly visible in the reflectivity spectra, shown in Figure 2 [not included here], as a sharp increase and decrease of the reflectivity near the atomic resonance. Intriguingly, the characteristic peak-and-trough behavior of atoms (seen at 370 K) was turned upside down at the highest temperature (420 K), where the cesium resonance was on the red side of the opal’s stopgap.
In nanophotonics, many efforts are currently being devoted to create arrays of nanoresonators in photonic crystals, for exquisite optical signal control on a chip. Unfortunately, however, there is a major challenge in engineering high-quality photonic resonators: they are all different due to inevitable fabrication variations. Hence, it is difficult to tune every resonator in sync. “Our atoms in the opal may be considered as the equivalent of an carefully engineered array of nano-resonators” explains Willem Vos, “Nature takes care that all resonators are all exactly the same. Our hybrid system solves the variability problem and could perhaps be used to make photonic memories, sensors or switches that are naturally tuned.” And leading Spanish theorist Javier Garcia de Abajo (ICFO) enthuses: “This is a fine and exciting piece of work, initiating the study of atomic resonances with photonic modes in a genuinely new fashion, and suggesting many exciting possibilities, for example through the extension of this study towards combinations with metal nanoplasmonics.”
Here’s a link to and a citation for the paper published in Physical Review B,
Nanophotonic hybridization of narrow atomic cesium resonances and photonic stop gaps of opaline nanostructures by Philip J. Harding, Pepijn W. H. Pinkse, Allard P. Mosk, and Willem L. Vos. Phys. Rev. B 91, 045123 – Published 20 January 2015 DOI: http://dx.doi.org/10.1103/PhysRevB.91.045123
This paper is behind a paywall but there is an earlier iteration of the paper available on the open access arXiv.org website operated by Cornell University,
Nanophotonic hybridization of narrow atomic cesium resonances and photonic stop gaps of opaline nanostructures by Philip J. Harding, Pepijn W.H. Pinkse, Allard P. Mosk, Willem L. Vos. (Submitted on 11 Sep 2014) arXiv:1409.3417
As I understand it, the arXiv.org website is intended to open up access to research and to offer an informal peer review process.
Finally, for anyone who’s nostalgic or perhaps has never heard Diana Ross sing ‘Upside Down’,