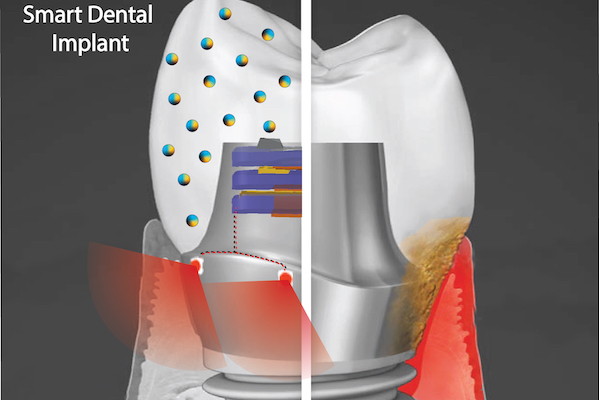
A September 9, 2021 news item on ScienceDaily announces research into ‘smart’ dental implants,
More than 3 million people in America have dental implants, used to replace a tooth lost to decay, gum disease, or injury. Implants represent a leap of progress over dentures or bridges, fitting much more securely and designed to last 20 years or more.
But often implants fall short of that expectation, instead needing replacement in five to 10 years due to local inflammation or gum disease, necessitating a repeat of a costly and invasive procedure for patients.
“We wanted to address this issue, and so we came up with an innovative new implant,” says Geelsu Hwang, an assistant professor in the University of Pennsylvania School of Dental Medicine, who has a background in engineering that he brings to his research on oral health issues.
The novel implant would implement two key technologies, Hwang says. One is a nanoparticle-infused material that resists bacterial colonization. And the second is an embedded light source to conduct phototherapy, powered by the natural motions of the mouth, such as chewing or toothbrushing. In a paper in the journal ACS Applied Materials & Interfaces and a 2020 paper in the journal Advanced Healthcare Materials, Hwang and colleagues lay out their platform, which could one day be integrated not only into dental implants but other technologies, such as joint replacements, as well.
…
A September 9, 2021 University of Pennsylvania news release (also on EurekAlert), which originated the news item, provides more technical details about the proposed technology,
“Phototherapy can address a diverse set of health issues,” says Hwang. “But once a biomaterial is implanted, it’s not practical to replace or recharge a battery. We are using a piezoelectric material, which can generate electrical power from natural oral motions to supply a light that can conduct phototherapy, and we find that it can successfully protect gingival tissue from bacterial challenge.”
In the paper, the material the researchers explored was barium titanate (BTO), which has piezoelectric properties that are leveraged in applications such as capacitators and transistors, but has not yet been explored as a foundation for anti-infectious implantable biomaterials. To test its potential as the foundation for a dental implant, the team first used discs embedded with nanoparticles of BTO and exposed them to Streptococcus mutans, a primary component of the bacterial biofilm responsible for tooth decay commonly known as dental plaque. They found that the discs resisted biofilm formation in a dose-dependent manner. Discs with higher concentrations of BTO were better at preventing biofilms from binding.
While earlier studies had suggested that BTO might kill bacteria outright using reactive oxygen species generated by light-catalyzed or electric polarization reactions, Hwang and colleagues did not find this to be the case due to the short-lived efficacy and off-target effects of these approaches. Instead, the material generates enhanced negative surface charge that repels the negatively charged cell walls of bacteria. It’s likely that this repulsion effect would be long-lasting, the researchers say.
“We wanted an implant material that could resist bacterial growth for a long time because bacterial challenges are not a one-time threat,” Hwang says.
The power-generating property of the material was sustained and in tests over time the material did not leach. It also demonstrated a level of mechanical strength comparable to other materials used in dental applications.
Finally, the material did not harm normal gingival tissue in the researchers’ experiments, supporting the idea that this could be used without ill effect in the mouth.
The technology is a finalist in the Science Center’s research accelerator program, the QED Proof-of-Concept program. As one of 12 finalists, Hwang and colleagues will receive guidance from experts in commercialization. If the project advances to be one of three finalists, the group has the potential to receive up to $200,000 in funding.
In future work, the team hopes to continue to refine the “smart” dental implant system, testing new material types and perhaps even using assymetric properties on each side of the implant components, one that encourages tissue integration on the side facing the gums and one that resists bacterial formation on the side facing the rest of the mouth.
“We hope to further develop the implant system and eventually see it commercialized so it can be used in the dental field,” Hwang says.
Here’s a link to and a citation for the paper,
Bimodal Nanocomposite Platform with Antibiofilm and Self-Powering Functionalities for Biomedical Applications by Atul Dhall, Sayemul Islam, Moonchul Park, Yu Zhang, Albert Kim, and Geelsu Hwang. ACS Appl. Mater. Interfaces 2021, 13, 34, 40379–40391 DOI: https://doi.org/10.1021/acsami.1c11791 Publication Date:August 18, 2021 Copyright © 2021 American Chemical Society
This paper is behind a paywall.
The work from 2020, mentioned in the news release, laid groundwork for the latest paper.
Human Oral Motion-Powered Smart Dental Implant (SDI) for In Situ Ambulatory Photo-biomodulation Therapy by Moonchul Park, Sayemul Islam, Hye-Eun Kim, Jonathan Korosto, Markus B. Blatz, Geelsu Hwang, and Albert Kim. Adv. Healthcare Mater. 2020, 9, 2000658 DOI: 10.1002/adhm.202000658 First published: 01 July 2020 © 2020 WILEY-VCH Verlag GmbH & Co. KGaA, WeinheimHuman
This paper is behind a paywall.