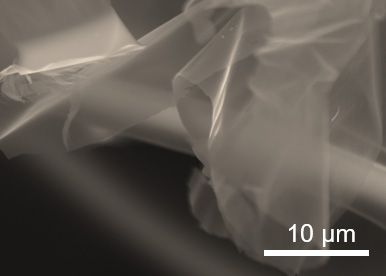
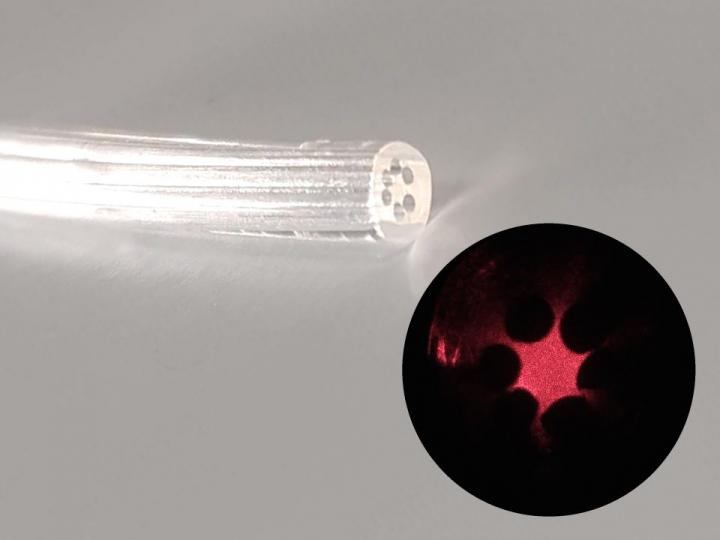
Apparently after you’ve finished imaging with your marine algae-based optical fibers, you can eat them. A July 24, 2020 news item on Nanowerk announces the new research,
An optical fiber made of agar has been produced at the University of Campinas (UNICAMP) in the state of São Paulo, Brazil. This device is edible, biocompatible and biodegradable. It can be used in vivo for body structure imaging, localized light delivery in phototherapy or optogenetics (e.g., stimulating neurons with light to study neural circuits in a living brain), and localized drug delivery.
Another possible application is the detection of microorganisms in specific organs, in which case the probe would be completely absorbed by the body after performing its function.
…
A July 24, 2020 Fundação de Amparo à Pesquisa dFundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) do Estado de São Paulo press release on EurekAlert, which originated the news item, provides a few more details about the researches and the work,
The research project, which was supported by São Paulo Research Foundation – FAPESP, was led by Eric Fujiwara, a professor in UNICAMP’s School of Mechanical Engineering, and Cristiano Cordeiro, a professor in UNICAMP’s Gleb Wataghin Institute of Physics, in collaboration with Hiromasa Oku, a professor at Gunma University in Japan.
An article on the study is published) in Scientific Reports, an online journal owned by Springer Nature.
Agar, also called agar-agar, is a natural gelatin obtained from marine algae. Its composition consists of a mixture of two polysaccharides, agarose and agaropectin. “Our optical fiber is an agar cylinder with an external diameter of 2.5 millimeters [mm] and a regular inner arrangement of six 0.5 mm cylindrical airholes around a solid core. Light is confined owing to the difference between the refraction indices of the agar core and the airholes,” Fujiwara told.
“To produce the fiber, we poured food-grade agar into a mold with six internal rods placed lengthwise around the main axis,” he continued. “The gel distributes itself to fill the available space. After cooling, the rods are removed to form airholes, and the solidified waveguide is released from the mold. The refraction index and geometry of the fiber can be adapted by varying the composition of the agar solution and mold design, respectively.”
The researchers tested the fiber in different media, from air and water to ethanol and acetone, concluding that it is context-sensitive. “The fact that the gel undergoes structural changes in response to variations in temperature, humidity and pH makes the fiber suitable for optical sensing,” Fujiwara said.
Another promising application is its simultaneous use as an optical sensor and a growth medium for microorganisms. “In this case, the waveguide can be designed as a disposable sample unit containing the necessary nutrients. The immobilized cells in the device would be optically sensed, and the signal would be analyzed using a camera or spectrometer,” he said.
###
About São Paulo Research Foundation (FAPESP)
The São Paulo Research Foundation (FAPESP) is a public institution with the mission of supporting scientific research in all fields of knowledge by awarding scholarships, fellowships and grants to investigators linked with higher education and research institutions in the State of São Paulo, Brazil. FAPESP is aware that the very best research can only be done by working with the best researchers internationally. Therefore, it has established partnerships with funding agencies, higher education, private companies, and research organizations in other countries known for the quality of their research and has been encouraging scientists funded by its grants to further develop their international collaboration. You can learn more about FAPESP at http://www.fapesp.br/en and visit FAPESP news agency at http://www.agencia.fapesp.br/en to keep updated with the latest scientific breakthroughs FAPESP helps achieve through its many programs, awards and research centers. You may also subscribe to FAPESP news agency at http://agencia.fapesp.br/subscribe.
As per my usual practice, here’s a link to and a citation for the paper,
Agarose-based structured optical fibre by Eric Fujiwara, Thiago D. Cabral, Miko Sato, Hiromasa Oku & Cristiano M. B. Cordeiro. Scientific Reports volume 10, Article number: 7035 (2020) DOI: https://doi.org/10.1038/s41598-020-64103-3 Published: 27 April 2020
This paper is open access.
Should you have a problem accessing the English language version of the FAPESP website, the Portuguese language version of the site seems more accessible (assuming you have the language skills).