Vancouver
This could be a first for Café Scientifique Vancouver. From a January 28, 2018 Café Scientifique Vancouver announcement (received via email)
This is a reminder that our next café with biotech entrepreneur Dr.Andrew Tait (TUESDAY, JANUARY 30TH [2018] at 7:30PM) in the back room of YAGGER'S DOWNTOWN (433 W Pender).
COMBINING TRADITIONAL NATURAL MEDICINES WITH SCIENTIFIC RESEARCH: UNVEILING THE POTENTIAL OF THE MANDARIN ORANGE PEEL
The orange peel is something most of us may think of as a throw-away compost item, but it is so much more. Travel back in time 9,000 years to China, where orange peel was found in the first fermented alcoholic beverage, and return to today, where mandarin orange peel remains one of China’s top selling herbs that promotes digestion. Now meet Tait Laboratories Inc., a company that was founded based on one chemistry Ph.D. student’s idea, that mandarin orange peel has the potential to reverse incurable neurodegenerative diseases like multiple sclerosis. You will learn about the company’s journey through a scientific lens, from its early days to the present, having developed a mandarin orange peel product sold across Canada in over 1,000 stores including 400 Rexall pharmacies. You will leave with a basic understanding of how herbal products like the company’s mandarin orange peel-based product are developed and brought to market in Canada, and about the science that is required to substantiate health claims on this and other exciting new botanical products.
Bio:
Dr. Andrew Tait is the founder of Tait Laboratories Inc., a company devoted to developing natural medicines from agricultural bi-products. After a B.Sc. in Biochemistry and M.Sc. in Chemistry from Concordia University (Montreal), he completed a Ph.D. in Chemistry at the
University of British Columbia [UBC].
Inspired by his thesis work on multiple sclerosis, he subsequently identified Traditional Chinese Medicines as having potential to treat a wide range of chronic diseases; he founded the company while finishing his graduate studies.
In 2012, he was invited to Ottawa to be awarded the NSERC [{Canada} Natural Sciences and Engineering Research Council] Innovation Challenge Award, for successfully translating his Ph.D. research to an entrepreneurial venture. In 2014, he was awarded the BC Food Processors Association “Rising Star” award.
Dr. Tait is a regularly invited speaker on the topics of entrepreneurship and the science supporting natural health products; he was keynote speaker in 2012 at the Annual Symposium of the Boucher Institute of Naturopathic Medicine (Vancouver) and in 2016 at the
Functional Foods and Natural Health Products Graduate Research Symposium (Winnipeg).
Supported by the Futurpreneur Canada, the Bank of Development of Canada, the UBC’s Entrepreneurship@UBC program, and the NSERC and NRC [{Canada} National Research Council] Industry Research Assistance Program (IRAP), he works with industrial and academic researchers developing safe, affordable, and clinically proven medicines. He successfully launched MS+ Mandarin Skin PlusÒ, a patent-pending digestive product now on shelf in over 1000 pharmacies and health food stores across Canada, including 400 Rexall pharmacies.
Dr. Tait mentors young companies as an Entrepreneur in Residence at both SFU [Simon Fraser University] Coast Capital Savings Venture Connection and also the Health Tech Innovation Hub and he also volunteers his time to mentor students of the Student Biotechnology Network.
Lest it be forgotten, many drugs and therapeutic agents are based on natural remedies; a fact often ignored in the discussion about drugs and natural remedies. In any event, I am surprised this talk is being hosted by Café Scientifique Vancouver which has tended to more ‘traditional’ (i.e., university academic) presentations without any hint of ‘alternative’ or ‘entrepreneurial’ aspects. I wonder if this is the harbinger of new things to come from the Café Scientifique Vancouver community.
Meanwhile, interested parties can find out more about Tait Laboratories on their company website. They are selling one product at this time (from the MS+ [Mandarin Skin Plus] product webpage,
MS+™ (Mandarin Skin Plus) is a revolutionary natural health product that aids with digestion and promotes gastrointestinal health. It is a patent-pending proprietary extract based on dry-aged mandarin orange peel, an ancient Traditional Chinese Medicine. This remedy has been safely used for centuries to relieve bloating, indigestion, diarrhea, nausea, upset stomach, cough with phlegm. Experience ULTIMATE DIGESTIVE RELIEF and top gastrointestinal health for only about a dollar a day!
Directions: take one capsule twice a day, up to six capsules per day. Swallow capsule directly OR dissolve powder in water.
60 vegan capsules for ~ 1 month supply
I would have liked to have seen a list of research papers and discussion of human clinical trials regarding their ‘digestive’ product. Will Tait be discussing his research and results into what seems to be a new direction (i.e., the use of mandarin skin peel-derived therapeutics for neurodegenerative diseases)?
I don’t think I’m going to make it to the talk but should anyone who attends care to answer the question, please feel free to add a comment.
ArtSci Salon in Toronto
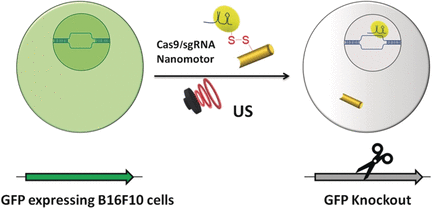
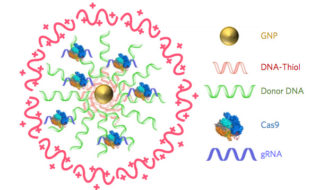
2018 is proving to be an active year for the ArtSci Salon folks in Toronto. They’ve just finished hosting a January 24-25, 2018 workshop and January 26, 2018 panel discussion on the gene-editing tool CRISPR/CAS9 (see my January 10, 2018 posting for a description).
Now they’ve announced another workshop and panel discussion on successive nights in February, the topic being: cells. From a January 29, 2018 ArtSci Salon announcement (received via email), Note: The panel discussion is listed first, then the workshop, then the artists’ biographies,
FROM CELL TO CANVAS: CREATIVE EXPLORATIONS OF THE MICROSCOPIC [panel discussion]
From the complex forms of the cell to the colonies created by the microbiota; from the undetectable chemical reactions activated by enzymes and natural processes to the environmental information captured through data visualization, the five local and international artists presenting tonight have developed a range of very diverse practices all inspired by the invisible, the undetectable and the microscopic.
We invite you to an evening of artist talks and discussion on the creative process of exploring the microscopic and using living organisms in art, on its potentials and implication for science and its popular dissemination, as well as on its ethics.
WITH:
Robyn Crouch
Mellissa Fisher
JULIA KROLIK
SHAVON MADDEN
TOSCA TERAN
FRIDAY, FEB 9, 2018
6:00-8:00 PM
THE FIELDS INSTITUTE
222 COLLEGE STREET,
RM 230
[Go to this page for access to registration]
FROM CELL TO CANVAS: CREATIVE EXPLORATIONS OF THE MICROSCOPIC [workshop]
THE EVENT WILL BE FOLLOWED BY A WORKSHOP BY: MELLISSA FISHER, SHAVON MADDEN AND JULIA KROLIK
FEB. 10, 2018
11:00AM-5:00PM
AT HACKLAB,
1266 Queen St West
[Go to this page for access to registration]
Workshop:
Design My Microbiome
Artist Mellissa Fisher invites participants to mould parts of her body in agar to create their own microbial version of her, alongside producing their own microbial portrait with painting techniques.
Cooking with the Invasive
Artist Shavon Madden invites participants to discuss invasive species like garlic mustard and cook invasive species whilst exploring, do species which we define and brand as invasive simply have no benefits?
Intoduction to Biological Staining
Artist & Scientist Julia Krolik invites participants to learn about 3 different types of biological staining and have a chance to try staining procedures.
BIOS:
ROBYN CROUCH
The symbolic imagery that comes through Robyn’s work invites one’s gaze inward to the cellular realms. There, one discovers playful depictions of chemical processes; the unseen lattice upon which our macrocosmic world is constructed. Technological advancements create windows into this molecular realm, and human consciousness acts as the interface between the seen and the unseen worlds. In her functional ceramic work, the influence of Chinese and Japanese tea ceremony encourages contemplation and appreciation of a quiet
moment. The viewer-participant can lose their train of thought while meandering through geometry and biota, connected by strands of double-helical DNA. A flash of recognition, a momentary mirror.
MELLISSA FISHER
Mellissa Fisher is a British Bio Artist based in Kent. Her practice explores the invisible world on our skin by using living organisms and by creating sculptures made with agar to show the public what the surface of our skin really looks like. She is best known for her work with bacteria and works extensively with collaborators in microbiology and immunology. She has exhibited an installation _ “Microbial Me”_with Professor Mark Clements and Dr Richard Harvey at The Eden Project for their permanent exhibition _“The Invisible You: The Human
Microbiome”._The installation included a living portrait in bacteria of the artists face as well as a time-lapse film of the sculpture growing.
JULIA KROLIK
Julia Krolik is a creative director, entrepreneur, scientist and award-winning artist. Her diverse background enables a rare cross-disciplinary empathy, and she continuously advocates for both art and science through several initiatives. Julia is the founder of Art the Science, a non-profit organization dedicated to facilitating artist residencies in scientific research laboratories to foster Canadian science-art culture and expand scientific knowledge communication to benefit the public. Through her consulting agency Pixels and Plans, Julia works with private and public organizations, helping them with strategy, data visualization and knowledge mobilization, often utilizing creative technology and skills-transfer workshops.
SHAVON MADDEN
Shavon Madden is a Brampton based artist, specializing in sculptural, performance and instillation based work exploring the social injustices inflicted on the environment and its creatures. Her work focuses on challenging social-environmental and political ethics, through the embodied experience and feelings of self. She graduated from the University of Toronto Specializing in Art and Art History, along with studies in Environmental Science and will be on her way to Edinburgh for her MFA. Shavon has had works shown at Shelly Peterson, the Burlington Art Gallery and the Art Gallery of Mississauga, among many others. Website: www.greenheartartistry.com [4]
TOSCA TERAN
Working with metal for over 30+ years, Tosca was introduced to glass as an artistic medium in 2004. Through developing bodies of work incorporating metal + glass Tosca has been awarded scholarships at The Corning Museum of Glass, Pilchuck Glass School and The Penland school of Crafts. Her work has been featured at SOFA New York, Culture Canada,
Metalsmith Magazine, The Toronto Design Exchange, and the Memphis Metal Museum. She has been awarded residencies at Gullkistan, Nes, and the Ayatana Research Program. A long-term guest artist instructor at the Ontario Science Centre, Tosca continues to explore materials, code, BioArt, SciArt and teach Metal + Glass courses out of her studio in Toronto.
It seems that these February events and the two events with Marta de Menezes are part of the FACTT (transdisciplinary and transnational festival of art and science) Toronto, from the FACTT Toronto webpage,
FACTT Toronto – Festival of Art & Science posted in: blog, events
The Arte Institute, in partnership with Cultivamos Cultura and ArtSi Salon, has the pleasure to announce FACTT – Festival of Art & Science in Toronto.
The Festival took place in Lisbon, New York, Mexico, Berlin and will continue in Toronto.
Exhibition: The Cabinet Project/ Art Sci Salon / FACTT
Artists:
Andrew Carnie
Elaine Whittaker
Erich Berger
Joana Ricou
Ken Rinaldo
Laura Beloff and Maria Antonia Gonzalez Valerio
Marta de Menezes and Luís Graça
Pedro Cruz
Dates: Jan 26- feb 15 [2018 {sic}]
Where: Meet us on Jan 26 [2018] in the Lobby of the Physics Department, 255 Huron Street
University of Toronto
When: 4:45 PM
You may want to keep an eye on the ArtSci Salon website although I find their posting schedule a bit erratic. Sometimes, I get email notices for events that aren’t yet listed on their website.