Tom Eldridge, a director and co-founder of Fullerex, has written a Jan. 5, 2017 essay titled: Is China still leading the graphene race? for Nanotechnology Now. Before getting to the essay, here’s a bit more about Fullerex and Tom Eldridge’s qualifications. From Fullerex’s LinkedIn description,
Fullerex is a leading independent broker of nanomaterials and nano-intermediates. Our mission is to support the advancement of nanotechnology in creating radical, transformative and sustainable improvement to society. We are dedicated to achieving these aims by accelerating the commercialisation and usage of nanomaterials across industry and beyond. Fullerex is active in market development and physical trading of advanced materials. We generate demand for nanomaterials across synergistic markets by stimulating innovation with end-users and ensuring robust supply chains are in place to address the growing commercial trade interest. Our end-user markets include Polymers and Polymer Composites, Coatings, Tyre and Rubber, Cementitious Composites, 3D Printing and Printed Electronics, the Energy sector, Lubricating Oils and Functional Fluids. The materials we cover: Nanomaterials: Includes fullerenes, carbon nanotubes and graphene, metal and metal oxide nanoparticles, and organic-inorganic hybrids. Supplied as raw nanopowders or ready-to-use dispersions and concentrates. Nano-intermediates: Producer goods and semi-finished products such as nano-enabled coatings, polymer masterbatches, conductive inks, thermal interface materials and catalysts.
As for Tom Eldridge, here’s more about him, his brother, and the company from the Fullerex About page,
Fullerex was founded by Joe and Tom Eldridge, brothers with a keen interest in nanotechnology and the associated emerging market for nanomaterials.
Joe has a strong background in trading with nearly 10 years’ experience as a stockbroker, managing client accounts for European Equities and FX. At University he read Mathematics at Imperial College London gaining a BSc degree and has closely followed the markets for disruptive technologies and advanced materials for a number of years.
Tom worked in the City of London for 7 years in commercial roles throughout his professional career, with an expertise in market data, financial and regulatory news. In his academic background, he earned a BSc degree in Physics and Philosophy at Kings College London and is a member of the Institute of Physics.
As a result, Fullerex has the strong management composition that allows the company to support the growth of the nascent and highly promising nanomaterials industry. Fullerex is a flexible company with drive, enthusiasm and experience, committed to aiding the development of this market.
Getting back to the matter at hand, that’s a rather provocative title for Tom Eldridge’s essay,. given that he’s a Brit and (I believe) the Brits viewed themselves as leaders in the ‘graphene race’ but he offers a more nuanced analysis than might be expected from the title. First, the patent landscape (from Eldridge’s Jan. 5, 2017 essay),
As competition to exploit the “wonder material” has intensified around the world, detailed reports have so far been published which set out an in-depth depiction of the global patent landscape for graphene, notably from CambridgeIP and the UK Intellectual Property Office, in 2013 and 2015 respectively. Ostensibly the number of patents and patent applications both indicated that China was leading the innovation in graphene technology. However, on closer inspection it became less clear as to how closely the patent figures themselves reflect actual progress and whether this will translate into real economic impact. Some of the main reasons to be doubtful included:
– 98% of the Chinese patent applications only cover China, so therefore have no worldwide monopoly.
– A large number of the Chinese patents are filed in December, possibly due to demand to meet patent quotas. The implication being that the patent filings follow a politically driven agenda, rather than a purely innovation or commercially driven agenda.
– In general, inventors could be more likely to file for patent protection in some countries rather than others e.g. for tax purposes. Which therefore does not give a truly accurate picture of where all the actual research activity is based.
– Measuring the proportion of graphene related patents to overall patents is more indicative of graphene specialisation, which shows that Singapore has the largest proportion of graphene patents, followed by China, then South Korea.
(Intellectual Property Office, 2015), (Ellis, 2015), (CambridgeIP, 2013)
Then, there’s the question of production,
Following the recent launch of the latest edition of the Bulk Graphene Pricing Report, which is available exclusively through The Graphene Council, Fullerex has updated its comprehensive list of graphene producers worldwide, and below is a summary of the number of graphene producers by country in 2017.
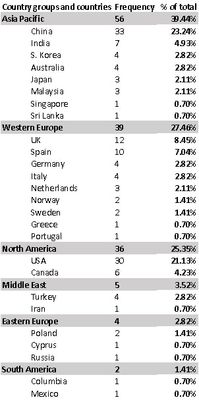 |
| Summary Table Showing the Number of Graphene Producers by Country and Region |
The total number of graphene producers identified is 142, across 27 countries. This research expands upon previous surveys of the graphene industry, such as the big data analysis performed by Nesta in 2015 (Shapira, 2015). The study by Nesta [formerly NESTA, National Endowment for Science, Technology and the Arts) is an independent charity that works to increase the innovation capacity of the UK; see Wikipedia here for more about NESTA] revealed 65 producers throughout 16 countries but was unable to glean accurate data on producers in Asia, particularly China.
As we can now see however from the data collected by Fullerex, China has the largest number of graphene producers, followed by the USA, and then the UK.
In addition to having more companies active in the production and sale of graphene than any other country, China also holds about 2/3rds of the global production capacity, according to Fullerex.
Eldridge goes on to note that the ‘graphene industry’ won’t truly grow and develop until there are substantive applications for the material. He also suggests taking another look at the production figures,
As with the patent landscape, rather than looking at the absolute figures, we can review the numbers in relative terms. For instance, if we normalise to account for the differences in the size of each country, by looking at the number of producers as a proportion of GDP, we see the following: Spain (7.18), UK (4.48), India (3.73), China (3.57), Canada (3.28) [emphasis mine], USA (1.79) (United Nations, 2013).
Unsurprisingly, each leading country has a national strategy for economic development which involves graphene prominently.
For instance, The Spanish Council for Scientific Research has lent 9 of its institutes along with 10 universities and other public R&D labs involved in coordinating graphene projects with industry.
The Natural Sciences and Engineering Research Council of Canada [NSERC] has placed graphene as one of five research topics in its target area of “Advanced Manufacturing” for Strategic Partnership Grants.
The UK government highlights advanced materials as one of its Eight Great Technologies, within which graphene is a major part of, having received investment for the NGI and GEIC buildings, along with EPSRC and Innovate UK projects. I wrote previously about the UK punching above its weight in terms of research, ( http://fullerex.com/index.php/articles/130-the-uk-needs-an-industrial-revolution-can-graphene-deliver/ ) but that R&D spending relative to GDP was too low compared to other developed nations. It is good to see that investment into graphene production in the UK is bucking that trend, and we should anticipate this will provide a positive economic outcome.
Yes, I’m particularly interested in the fact Canada becomes more important as a producer when the numbers are relative but it is interesting to compare the chart with Eldridge’s text and to note how importance shifts depending on what numbers are being considered.
I recommend reading Eldridge’s piece in its entirety.
A few notes about graphene in Canada
By the way, the information in Eldridge’s essay about NSERC’s placement of graphene as a target area for grants is news to me. (As I have often noted here, I get more information about the Canadian nano scene from international sources than I do from our national sources.)
Happily I do get some home news such as a Jan. 5, 2017 email update from Lomiko Metals, a Canadian junior exploration company focused on graphite and lithium. The email provides the latest information from the company (as I’m not an expert in business or mining this is not an endorsement),
On December 13, 2016 we were excited to announce the completion of our drill program at the La Loutre flake graphite property. We received very positive results from our 1550 meter drilling program in 2015 in the area we are drilling now. In that release I stated, ”The intercepts of multiple zones of mineralization in the Refractory Zone where we have reported high grade intercepts previously is a very promising sign. The samples have been rushed to the ALS Laboratory for full assay testing,” We hope to have the results of those assays shortly.
December 16, 2016 Lomiko announced a 10:1 roll back of our shares. We believe that this roll back is important as we work towards securing long term equity financing for the company. Lomiko began trading on the basis of the roll back on December 19.
We believe that Graphite has a bright future because of the many new products that will rely on the material. I have attached a link to a video on Lomiko, Graphite and Graphene.
https://youtu.be/Y–Y_Ub6oC4
January 3, 2017 Lomiko announced the extension and modification of its option agreements with Canadian Strategic Metals Inc. for the La Loutre and Lac des Iles properties. The effect of this extension is to give Lomiko additional time to complete the required work under the agreements.
Going forward Lomiko is in a much stronger position as the result of our share roll back. Potential equity funders who are very interested in our forthcoming assay results from La Loutre and the overall prospects of the company, have been reassured by our share consolidation.
Looking forward to 2017, we anticipate the assays of the La Loutre drilling to be delivered in the next 90 days, sooner we hope. We also anticipate additional equity funding will become available for the further exploration and delineation of the La Loutre and Lac des Iles properties and deposits.
More generally, we are confident that the market for large flake graphite will become firmer in 2017. Lomikos strategy of identifying near surface, ready to mine, graphite nodes puts us in the position to take advantage of improvements in the graphite price without having to commit large sums to massive mine development. As we identify and analyze the graphite nodes we are finding we increase the potential resources of the company. 2017 should see significantly improved resource estimates for Lomikos properties.
As I wasn’t familiar with the term ‘roll back of shares’, I looked it up and found this in an April 18, 2012 posting by Dudley Pierce Baker on kitco.com,
As a general rule, we hate to see an announcement of a share rollback, however, there exceptions which we cover below. Investors should always be aware that if a company has, say over 150 million shares outstanding, in our opinion, it is a potential candidate for a rollback and the announcement should not come as a surprise.
Weak markets, a low share price, a large number of shares outstanding, little or no cash and you have a company which is an idea candidate for a rollback.
The basic concept of a rollback or consolidation in a company’s shares is rather simple.
…
We are witnessing a few cases of rollbacks not with the purpose of raising more money but rather to facilitate the listing of the company’s shares on the NYSE [New York Stock Exchange] Amex.
…
I have no idea what situation Lomiko finds itself in but it should be noted that graphere research has been active since 2004 when the first graphene sheets were extracted from graphite. This is a relatively new field of endeavour and Lomiko (along with other companies) is in the position of pioneering the effort here in Canada. That said, there are many competitors to graphene and major international race to commercialize nanotechnology-enabled products.
Are there any leaders in the ‘graphene race?
Getting back to the question in the headline, I don’t think there are any leaders at the moment. No one seems to have what they used to call “a killer app,” that one application/product that everyone wants and which drive demand for graphene.