A December 18, 2023 news item on ScienceDaily shifted my focus from hardware to software when considering memory in brainlike (neuromorphic) computing,
An interdisciplinary team consisting of researchers from the Center for Cognition and Sociality and the Data Science Group within the Institute for Basic Science (IBS) [Korea] revealed a striking similarity between the memory processing of artificial intelligence (AI) models and the hippocampus of the human brain. This new finding provides a novel perspective on memory consolidation, which is a process that transforms short-term memories into long-term ones, in AI systems.
…
A November 28 (?), 2023 IBS press release (also on EurekAlert but published December 18, 2023, which originated the news item, describes how the team went about its research,
In the race towards developing Artificial General Intelligence (AGI), with influential entities like OpenAI and Google DeepMind leading the way, understanding and replicating human-like intelligence has become an important research interest. Central to these technological advancements is the Transformer model [Figure 1], whose fundamental principles are now being explored in new depth.
The key to powerful AI systems is grasping how they learn and remember information. The team applied principles of human brain learning, specifically concentrating on memory consolidation through the NMDA receptor in the hippocampus, to AI models.
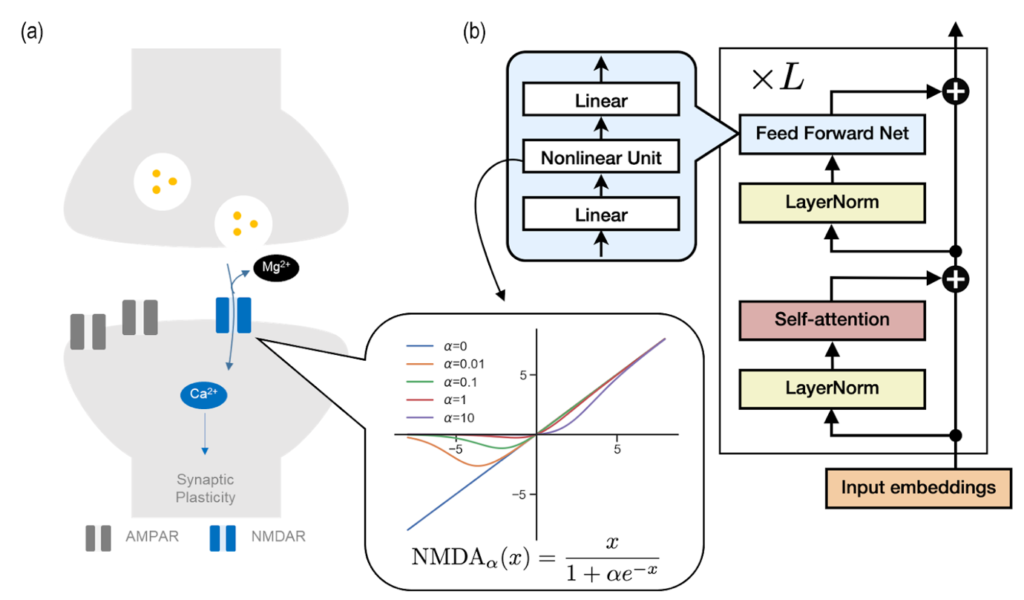
The NMDA receptor is like a smart door in your brain that facilitates learning and memory formation. When a brain chemical called glutamate is present, the nerve cell undergoes excitation. On the other hand, a magnesium ion acts as a small gatekeeper blocking the door. Only when this ionic gatekeeper steps aside, substances are allowed to flow into the cell. This is the process that allows the brain to create and keep memories, and the gatekeeper’s (the magnesium ion) role in the whole process is quite specific.
The team made a fascinating discovery: the Transformer model seems to use a gatekeeping process similar to the brain’s NMDA receptor [see Figure 1]. This revelation led the researchers to investigate if the Transformer’s memory consolidation can be controlled by a mechanism similar to the NMDA receptor’s gating process.
In the animal brain, a low magnesium level is known to weaken memory function. The researchers found that long-term memory in Transformer can be improved by mimicking the NMDA receptor. Just like in the brain, where changing magnesium levels affect memory strength, tweaking the Transformer’s parameters to reflect the gating action of the NMDA receptor led to enhanced memory in the AI model. This breakthrough finding suggests that how AI models learn can be explained with established knowledge in neuroscience.
C. Justin LEE, who is a neuroscientist director at the institute, said, “This research makes a crucial step in advancing AI and neuroscience. It allows us to delve deeper into the brain’s operating principles and develop more advanced AI systems based on these insights.”
CHA Meeyoung, who is a data scientist in the team and at KAIST [Korea Advanced Institute of Science and Technology], notes, “The human brain is remarkable in how it operates with minimal energy, unlike the large AI models that need immense resources. Our work opens up new possibilities for low-cost, high-performance AI systems that learn and remember information like humans.”
What sets this study apart is its initiative to incorporate brain-inspired nonlinearity into an AI construct, signifying a significant advancement in simulating human-like memory consolidation. The convergence of human cognitive mechanisms and AI design not only holds promise for creating low-cost, high-performance AI systems but also provides valuable insights into the workings of the brain through AI models.
This research was presented at the 37th Conference on Neural Information Processing Systems (NeurIPS 2023) before being published in the proceedings, I found a PDF of the presentation and an early online copy of the paper before locating the paper in the published proceedings.
PDF of presentation: Transformer as a hippocampal memory consolidation model based on NMDAR-inspired nonlinearity
PDF copy of paper:
Transformer as a hippocampal memory consolidation model based on NMDAR-inspired nonlinearity by Dong-Kyum Kim, Jea Kwon, Meeyoung Cha, C. Justin Lee.
This paper was made available on OpenReview.net:
OpenReview is a platform for open peer review, open publishing, open access, open discussion, open recommendations, open directory, open API and open source.
It’s not clear to me if this paper is finalized or not and I don’t know if its presence on OpenReview constitutes publication.
Finally, the paper published in the proceedings,
Transformer as a hippocampal memory consolidation model based on NMDAR-inspired nonlinearity by Dong Kyum Kim, Jea Kwon, Meeyoung Cha, C. Justin Lee. Part of Advances in Neural Information Processing Systems 36 (NeurIPS 2023) Main Conference Track
This link will take you to the abstract, access the paper by clicking on the Paper tab.