It’s easy to forget how hard we are on our textiles. We rip them, step on them, agitate them in water, splatter them with mud, and more. So, what happens when we integrate batteries and electronics into them? An Oct. 20, 2016 news item on phys.org describes one of the latest ‘textile batter technologies’,
Electronics that can be embedded in clothing are a growing trend. However, power sources remain a problem. In the journal Angewandte Chemie, scientists have now introduced thin, flexible, lithium ion batteries with self-healing properties that can be safely worn on the body. Even after completely breaking apart, the battery can grow back together without significant impact on its electrochemical properties.
An Oct. 20, 2016 Wiley Angewandte Chemie International Edition press release (also on EurekAlert), which originated the news item, describes some of the problems associated with lithium-ion batteries and this new technology designed to address them,
Existing lithium ion batteries for wearable electronics can be bent and rolled up without any problems, but can break when they are twisted too far or accidentally stepped on—which can happen often when being worn. This damage not only causes the battery to fail, it can also cause a safety problem: Flammable, toxic, or corrosive gases or liquids may leak out.
A team led by Yonggang Wang and Huisheng Peng has now developed a new family of lithium ion batteries that can overcome such accidents thanks to their amazing self-healing powers. In order for a complicated object like a battery to be made self-healing, all of its individual components must also be self-healing. The scientists from Fudan University (Shanghai, China), the Samsung Advanced Institute of Technology (South Korea), and the Samsung R&D Institute China, have now been able to accomplish this.
The electrodes in these batteries consist of layers of parallel carbon nanotubes. Between the layers, the scientists embedded the necessary lithium compounds in nanoparticle form (LiMn2O4 for one electrode, LiTi2(PO4)3 for the other). In contrast to conventional lithium ion batteries, the lithium compounds cannot leak out of the electrodes, either while in use or after a break. The thin layer electrodes are each fixed on a substrate of self-healing polymer. Between the electrodes is a novel, solvent-free electrolyte made from a cellulose-based gel with an aqueous lithium sulfate solution embedded in it. This gel electrolyte also serves as a separation layer between the electrodes.
After a break, it is only necessary to press the broken ends together for a few seconds for them to grow back together. Both the self-healing polymer and the carbon nanotubes “stick” back together perfectly. The parallel arrangement of the nanotubes allows them to come together much better than layers of disordered carbon nanotubes. The electrolyte also poses no problems. Whereas conventional electrolytes decompose immediately upon exposure to air, the new gel is stable. Free of organic solvents, it is neither flammable nor toxic, making it safe for this application.
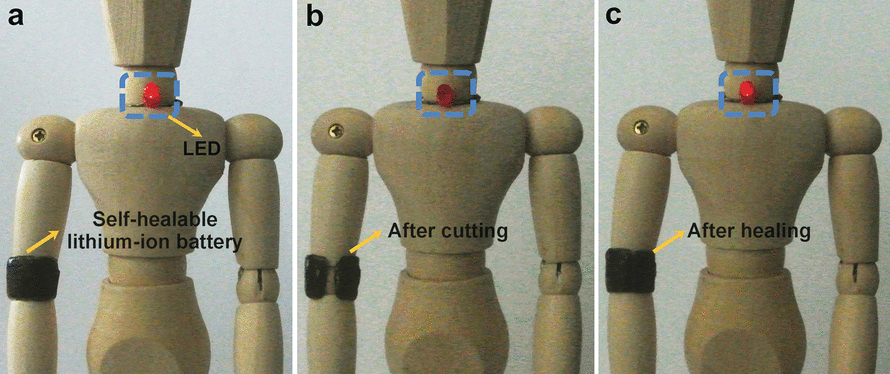
The capacity and charging/discharging properties of a battery “armband” placed around a doll’s elbow were maintained, even after repeated break/self-healing cycles.
Here’s a link to and a citation for the paper,
A Self-Healing Aqueous Lithium-Ion Battery by Yang Zhao, Ye Zhang, Hao Sun, Xiaoli Dong, Jingyu Cao, Lie Wang, Yifan Xu, Jing Ren, Yunil Hwang, Dr. In Hyuk Son, Dr. Xianliang Huang, Prof. Yonggang Wang, and Prof. Huisheng Peng. Angewandte Chemie International Edition DOI: 10.1002/anie.201607951 Version of Record online: 12 OCT 2016
© 2016 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
This paper is behind a paywall.