A January 11, 2023 news item on ScienceDaily announces some new research on cellulose nanocrystals (CNC),
Cellulose nanocrystals—bio-based nanomaterials derived from natural resources such as plant cellulose—are valuable for their use in water treatment, packaging, tissue engineering, electronics, antibacterial coatings and much more. Though the materials provide a sustainable alternative to non-bio-based materials, transporting them in liquid taxes industrial infrastructures and leads to environmental impacts.
A team of Penn State [Pennsylvania State University] chemical engineering researchers studied the mechanisms of drying the nanocrystals and proposed nanotechnology to render the nanocrystals highly redispersible in aqueous mediums, while retaining their full functionality, to make them easier to store and transport. They published their results in the journal Biomacromolecules.
This image illustrates what the drying process does,
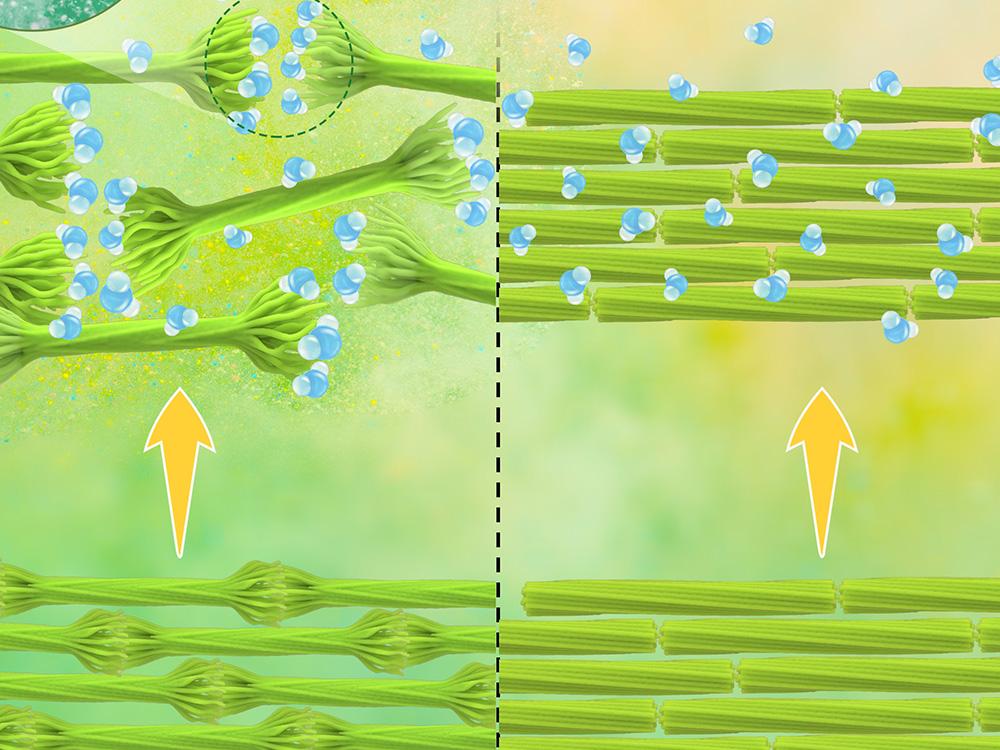
A Pennsylvania State University (Penn State) news release (also on EurekAlert) by Mariah R. Lucas, which originated the news item, provides more detail, Note: A link has been removed,
“We looked at how we could take hairy nanocrystals, dry them in ovens, and redisperse them in solutions containing different ions,” said co-first author Breanna Huntington, current chemical engineering doctoral student at the University of Delaware and former member of the Sheikhi Research Group while an undergraduate student at Penn State. “We then compared their functionality to conventional, non-hairy cellulose nanocrystals.”
The nanocrystals have negatively charged cellulose chains at their ends, known as hairs. When rehydrated, the hairs repel each other and separate, dispersing again through a liquid, as a result of electrosteric repulsion — a term meaning charge-driven, or electrostatic, and free-volume dependent, or steric.
“The hairy ends of the nanocrystals are nanoengineered to be negatively charged and repel each other when placed in an aqueous medium,” said corresponding author Amir Sheikhi, Penn State assistant professor of chemical engineering and of biomedical engineering. “To have maximum function, the nanocrystals must be separate, individual particles, not chained together as they are when they are dry.”
After the hairy particles were redispersed, researchers tested them and measured their size and surface properties and found their characteristics and performance were the same as those that had never been dried. They also found the particles could perform well and maintain their stability in a variety of liquid mixtures of different salinities and pH levels.
“The hairy nanocrystals can become redispersed even at high salt concentrations, which is convenient, as they remain functional in harsh media and may be used in a broad range of applications,” said co-first author Mica Pitcher, Penn State doctoral student in chemistry, supervised by Sheikhi. “This work may pave the way for sustainable and large-scale processing of nanocelluloses without using additive or energy-intensive methods.”
The Penn State College of Engineering Summer Research Experiences for Undergraduates program and the NASA Pennsylvania Space Grant Consortium graduate fellowship program supported this work.
Here’s a link to and a citation for the paper,
Nanoengineering the Redispersibility of Cellulose Nanocrystals by Breanna Huntington, Mica L. Pitcher, and Amir Sheikhi. Biomacromolecules 2023, 24, 1, 43–56 DOI: https://doi.org/10.1021/acs.biomac.2c00518 Publication Date:December 5, 2022 Copyright © 2022 American Chemical Society
This paper is behind a paywall.