I am contrasting two very different studies on silver nanoparticles in water and their effect on the environment to highlight the complex nature of determining the risks and environmental effects associated with nanoparticles in general. One piece of research suggests that silver nanoparticles are less dangerous than other commonly used forms of silver while the other piece raises some serious concerns.
A Feb. 28, 2013 news item on Nanowerk features research about the effects that silver nanoparticles have on aquatic ecosystems (Note: A link has been removed),
According to Finnish-Estonian joint research with data obtained on two crustacean species, there is apparently no reason to consider silver nanoparticles more dangerous for aquatic ecosystems than silver ions.
The results were reported in the journal Environmental Science and Pollution Research late last year (“Toxicity of two types of silver nanoparticles to aquatic crustaceans Daphnia magna and Thamnocephalus platyurus”). Jukka Niskanen has utilised the same polymerisation and coupling reactions in his doctoral dissertation studying several hybrid nanomaterials, i.e. combinations of synthetic polymers and inorganic (gold, silver and montmorillonite) nanoparticles. Niskanen will defend his doctoral thesis at the University of Helsinki in April.
The University of Helsikinki Feb. 28, 2013 press release written by Minna Merilainen and which originated the new item provides details about the research,
“Due to the fact that silver in nanoparticle form is bactericidal and also fungicidal and also prevents the reproduction of those organisms, it is now used in various consumer goods ranging from wound dressing products to sportswear,” says Jukka Niskanen from the Laboratory of Polymer Chemistry at the University of Helsinki, Finland.A joint study from the University of Helsinki and the National Institute of Chemical Physics and Biophysics (Tallinn, Estonia), Toxicity of two types of silver nanoparticles to aquatic crustaceans Daphnia magna and Thamnocephalus platyurus, shows that silver nanoparticles are apparently no more hazardous to aquatic ecosystems than a water-soluble silver salt. The study compared the ecotoxicity of silver nanoparticles and a water-soluble silver salt.
“Our conclusion was that the environmental risks caused by silver nanoparticles are seemingly not higher than those caused by a silver salt. However, more research is required to reach a clear understanding of the safety of silver-containing particles,” Niskanen says.
Indeed, silver nanoparticles were found to be ten times less toxic than the soluble silver nitrate - a soluble silver salt used for the comparison.
The bioavailability of silver varies in different test media
To explain this phenomenon, the researchers refer to the variance in the bioavailability of silver to crustaceans in different tested media.
University lecturer Olli-Pekka Penttinen from the Department of Environmental Sciences of the University of Helsinki goes on to note that the inorganic and organic compounds dissolved in natural waters (such as humus), water hardness and sulfides have a definite impact on the bioavailability of silver. Due to this, the toxicity of both types of tested nanoparticles and the silver nitrate measured in the course of the study was lower in natural water than in artificial fresh water.
The toxicity of silver nanoparticles and silver ions was studied using two aquatic crustaceans, a water flea (Daphnia magna) and a fairy shrimp ( Thamnocephalus platyurus). Commercially available protein-stabilised particles and particles coated with a water-soluble, non-toxic polymer, specifically synthesised for the purpose, were used in the study. First, the polymers were produced utilising a controlled radical polymerization method. Synthetic polymer-grafted silver particles were then produced by attaching the water-soluble polymer to the surface of the silver with a sulfur bond.
Jukka Niskanen has utilised such polymerisation and coupling reactions in his doctoral dissertation. Polymeric and hybrid materials: polymers on particle surfaces and air-water interfaces, studying several hybrid nanomaterials , i.e., combinations of synthetic polymers and inorganic (gold, silver and montmorillonite) nanoparticles....
It was previously known from other studies and research results that silver changes the functioning of proteins and enzymes. It has also been shown that silver ions can prevent the replication of DNA. Concerning silver nanoparticles, tests conducted on various species of bacteria and fungi have indicated that their toxicity varies. For example, gram-negative bacteria such as Escherichia coli are more sensitive to silver nanoparticles than gram-positive ones (such as Staphylococcus aureus). The difference in sensitivity is caused by the structural differences of the cell membranes of the bacteria. The cellular toxicity of silver nanoparticles in mammals has been studied as well. It has been suggested that silver nanoparticles enter cells via endocytosis and then function in the same manner as in bacterial cells, damaging DNA and hindering cell respiration. Electron microscope studies have shown that human skin is permeable to silver nanoparticles and that the permeability of damaged skin is up to four times higher than that of healthy skin.
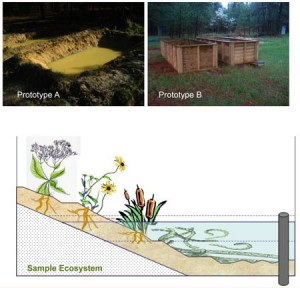
While this Finnish-Estonian study suggests that silver nanoparticles do not have a negative impact on the tested crustaceans in an aquatic environment, there’s a study from Duke University suggests that silver nanoparticles in wastewater which is later put to agricultural use may cause problems. From the Feb. 27, 2013 news release on EurekAlert,
In experiments mimicking a natural environment, Duke University researchers have demonstrated that the silver nanoparticles used in many consumer products can have an adverse effect on plants and microorganisms.
…
The main route by which these particles enter the environment is as a by-product of water and sewage treatment plants. [emphasis] The nanoparticles are too small to be filtered out, so they and other materials end up in the resulting “sludge,” which is then spread on the land surface as a fertilizer.
…
The researchers found that one of the plants studied, a common annual grass known as Microstegium vimeneum, had 32 percent less biomass in the mesocosms treated with the nanoparticles. Microbes were also affected by the nanoparticles, Colman [Benjamin Colman, a post-doctoral fellow in Duke’s biology department and a member of the Center for the Environmental Implications of Nanotechnology (CEINT)] said. One enzyme associated with helping microbes deal with external stresses was 52 percent less active, while another enzyme that helps regulate processes within the cell was 27 percent less active. The overall biomass of the microbes was also 35 percent lower, he said.
“Our field studies show adverse responses of plants and microorganisms following a single low dose of silver nanoparticles applied by a sewage biosolid,” Colman said. “An estimated 60 percent of the average 5.6 million tons of biosolids produced each year is applied to the land for various reasons, and this practice represents an important and understudied route of exposure of natural ecosystems to engineered nanoparticles.”
“Our results show that silver nanoparticles in the biosolids, added at concentrations that would be expected, caused ecosystem-level impacts,” Colman said. “Specifically, the nanoparticles led to an increase in nitrous oxide fluxes, changes in microbial community composition, biomass, and extracellular enzyme activity, as well as species-specific effects on the above-ground vegetation.”
As previously noted, these two studies show just how complex the questions of risk and nanoparticles can become. You can find out more about the Finish-Estonian study,
Toxicity of two types of silver nanoparticles to aquatic crustaceans Daphnia magna and Thamnocephalus platyurus by Irina Blinova, Jukka Niskanen, Paula Kajankari, Liina Kanarbik, Aleksandr Käkinen, Heikki Tenhu, Olli-Pekka Penttinen, and Anne Kahru. Environmental Science and Pollution Research published November 11, 2012 online
The publisher offers an interesting option for this article. While it is behind a paywall, access is permitted through a temporary window if you want to preview a portion of the article that lies beyond the abstract.
Meanwhile here’s the article by the Duke researchers,
Low Concentrations of Silver Nanoparticles in Biosolids Cause Adverse Ecosystem Responses under Realistic Field Scenario by Benjamin P. Colman, Christina L. Arnaout, Sarah Anciaux, Claudia K. Gunsch, Michael F. Hochella Jr, Bojeong Kim, Gregory V. Lowry, Bonnie M. McGill, Brian C. Reinsch, Curtis J. Richardson, Jason M. Unrine, Justin P. Wright, Liyan Yin, and Emily S. Bernhardt. PLoS ONE 2013; 8 (2): e57189 DOI: 10.1371/journal.pone.0057189
This article is open access as are all articles published by the Public Library of Science (PLoS) journals.
For anyone interested in the Duke University/CEINT mesocosm project, I made mention of it in an Aug. 15, 2011 posting.