Last week (specifically, Tuesday, March 3, 2020), someone moved away from me during a class. I’d sneezed.
The irony of the situation is that of the two of us, with my lung issues I’d be the one most at risk of getting very ill and/or dying from COVID-19. ([1] Yes, I confirmed that was the reason she’d moved. [2] The therapeutic nanoparticles news item is coming later) Here are the risk factors to take into account (from the US Centers for Disease Control’s People at Risk for Serious Illness from COVID-19 webpage,
- Older adults [Note: In one report the age range was stated as ‘people over 70’]
- People who have serious chronic medical conditions like:
- Heart disease
- Diabetes
- Lung disease
I’m not suggesting that all precautions be abandoned but it would seem that panic might not be called for. Jeremy Samuel Faust, an emergency medicine physician at Brigham and Women’s Hospital in Boston, faculty in its division of health policy and public health, and an instructor at Harvard Medical School, has written a calming March 4, 2020 article (COVID-19 Isn’t As Deadly As We Think; Don’t hoard masks and food. Figure out how to help seniors and the immunosuppressed stay healthy.) for Slate.com (Note: Links have been removed],
There are many compelling reasons to conclude that SARS-CoV-2, the virus that causes COVID-19, is not nearly as deadly as is currently feared. But COVID-19 panic has set in nonetheless. You can’t find hand sanitizer in stores, and N95 face masks are being sold online for exorbitant prices, never mind that neither is the best way to protect against the virus (yes, just wash your hands). The public is behaving as if this epidemic is the next Spanish flu, which is frankly understandable given that initial reports have staked COVID-19 mortality at about 2–3 percent, quite similar to the 1918 pandemic that killed tens of millions of people.
Allow me to be the bearer of good news. These frightening numbers are unlikely to hold. The true case fatality rate, known as CFR, of this virus is likely to be far lower than current reports suggest. Even some lower estimates, such as the 1 percent death rate recently mentioned by the directors of the National Institutes of Health and the Centers for Disease Control and Prevention, likely substantially overstate the case. [emphases mine]
…
But the most straightforward and compelling evidence that the true case fatality rate of SARS-CoV-2 is well under 1 percent comes not from statistical trends and methodological massage, but from data from the Diamond Princess cruise outbreak and subsequent quarantine off the coast of Japan.
A quarantined boat is an ideal—if unfortunate—natural laboratory to study a virus. Many variables normally impossible to control are controlled. We know that all but one patient boarded the boat without the virus. We know that the other passengers were healthy enough to travel. We know their whereabouts and exposures. While the numbers coming out of China are scary, we don’t know how many of those patients were already ill for other reasons. How many were already hospitalized for another life-threatening illness and then caught the virus? How many were completely healthy, caught the virus, and developed a critical illness? In the real world, we just don’t know.
Here’s the problem with looking at mortality numbers in a general setting: In China, 9 million people die per year, which comes out to 25,000 people every single day, or around 1.5 million people over the past two months alone. A significant fraction of these deaths results from diseases like emphysema/COPD, lower respiratory infections, and cancers of the lung and airway whose symptoms are clinically indistinguishable from the nonspecific symptoms seen in severe COVID-19 cases. And, perhaps unsurprisingly, the death rate from COVID-19 in China spiked precisely among the same age groups in which these chronic diseases first become common. During the peak of the outbreak in China in January and early February, around 25 patients per day were dying with SARS-CoV-2. Most were older patients in whom the chronic diseases listed above are prevalent. Most deaths occurred in Hubei province, an area in which lung cancer and emphysema/COPD are significantly higher than national averages in China, a country where half of all men smoke. How were doctors supposed to sort out which of those 25 out of 25,000 daily deaths were solely due to coronavirus, and which were more complicated? What we need to know is how many excess deaths this virus causes.
…
This all suggests that COVID-19 is a relatively benign disease for most young people, and a potentially devastating one for the old and chronically ill, albeit not nearly as risky as reported. Given the low mortality rate among younger patients with coronavirus—zero in children 10 or younger among hundreds of cases in China, and 0.2-0.4 percent in most healthy nongeriatric adults (and this is still before accounting for what is likely to be a high number of undetected asymptomatic cases)—we need to divert our focus away from worrying about preventing systemic spread among healthy people—which is likely either inevitable, or out of our control—and commit most if not all of our resources toward protecting those truly at risk of developing critical illness and even death: everyone over 70, and people who are already at higher risk from this kind of virus.
This still largely comes down to hygiene and isolation. But in particular, we need to focus on the right people and the right places. Nursing homes, not schools. Hospitals, not planes. We need to up the hygienic and isolation ante primarily around the subset of people who can’t simply contract SARS-CoV-2 and ride it out the way healthy people should be able to.
…
Curtis Kim of Vancouver, Canada, has created a website dedicated to tracking the statistics and information about COVID-19 in Canada and around the world. Here’s more about Kim and the website from a March 8, 2020 article by Megan Devlin for the Daily Hive,
Curtis Kim, who studied Computer Systems Technology at the British Columbia Institute of Technology [BCIT], launched the site this week after getting frustrated he was spending so much time on various websites looking for daily coronavirus updates.
…
The site breaks down the number of cases in Canada, the number of deaths (zero in Canada so far), and the number of people who have recovered. Further down, it provides the same stats for global COVID-19 cases.
There’s also a colour-coded map showing where cases are distributed, and a feed of latest news articles about the virus. Kim also included information about symptoms and how to contact Canadian public health services.
…
Kim is looking for work and given what I’ve seen of his COVID-19 website, he should have no difficulty. Although I think it might be an idea for him to explain how the ‘lethality’ rate on his website has been obtained since Faust who seems to have more directly relevant experience suggests in his article that the numbers are highly problematic,
My name is Curtis, recently graduated from BCIT. I thought it would be a serious worldwide issue considering the speed of the spread of this virus ever since this COVID-19 occurred. I frequently googled to check up the current status by going through many websites and felt I was wasting time repeatedly searching with same keywords and for sure I wasn’t the only one feeling this way. That’s why I started creating this application. It provides up-to-date information on the COVID-19 broken by province and country around the world, key contact information, and latest news. I like to help people, and want them to understand this situation easily using this application. Hopefully this situation improves soon.
If you have any further inquries about the information on this web application, Please reach me at curtisk808@gmail.com
At about 11:45 am (PT) on March 9, 2020, Kim’s COVID-19 website was updated to include one death in Canada. As you might expect, ti was a resident in a long term care home. Wanyee Li’s March 9, 2020 article for The Star presents the news,
A resident at a long-term care home experiencing a COVID-19 outbreak in North Vancouver has died after contracting the virus, B.C. health officials confirmed Monday [March 9, 2020].
It is the first reported death in Canada linked to the virus.
The outbreak at the Lynn Valley Care Centre has so far been linked to three community transmission cases of the virus.
Provincial Health Officer Dr. Bonnie Henry confirmed five new cases of COVID-19 in B.C. on Monday [March 9, 2020], putting the total in the province at 32.
The five new cases include one health-care worker, two people who are close contacts of an existing case, one person who recently returned from travel to Iran and another who was in Italy recently.
Officials are conducting an investigation into the three community transmission cases at the long-term care home to determine how a health care worker contracted the virus.
…
I looked up the population figures for the province of British Columbia (BC; Wikipedia entry for Demographics of British Columbia). As of the 2016 census, there were 4,648,055 people in the province. Assuming that population number holds, 67 cases in all of Canada (with 27 cases in BC) of COVID-19 don’t seem like big numbers.
We should definitely take precautions and be careful but there’s no need to panic.
Nanoparticles and a COVID-19 treatment?
Don’t hold you breath. This March 5, 2020 news item on Nanowerk is speculative,
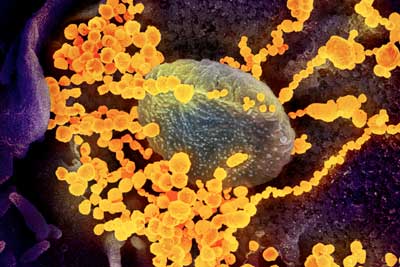
There is no vaccine or specific treatment for COVID-19, the disease caused by the severe acute respiratory syndrome coronavirus 2, or SARS-CoV-2.
Since the outbreak began in late 2019, researchers have been racing to learn more about SARS-CoV-2, which is a strain from a family of viruses known as coronavirus for their crown-like shape.
Northeastern Ûniversity] chemical engineer Thomas Webster, who specializes in developing nano-scale medicine and technology to treat diseases, is part of a contingency of scientists that are contributing ideas and technology to the Centers for Disease Control and Prevention to fight the COVID-19 outbreak.
The idea of using nanoparticles, Webster says, is that the virus behind COVID-19 consists of a structure of a similar scale as his nanoparticles. At that scale, matter is ultra-small, about ten thousand times smaller than the width of a single strand of hair.
..
A March 4, 2020 Northeastern University news release by Roberto Molar Candanosa, which originated the news item, delves further into Webster’s thinking process,
Webster is proposing particles of similar sizes that could attach to SARS-CoV-2 viruses, disrupting their structure with a combination of infrared light treatment. That structural change would then halt the ability of the virus to survive and reproduce in the body.
“You have to think in this size range,” says Webster, Art Zafiropoulo Chair of chemical engineering at Northeastern. “In the nanoscale size range, if you want to detect viruses, if you want to deactivate them.”
Finding and neutralizing viruses with nanomedicine is at the core of what Webster and other researchers call theranostics, which focuses on combining therapy and diagnosis. Using that approach, his lab has specialized in nanoparticles to fight the microbes that cause influenza and tuberculosis.
“It’s not just having one approach to detect whether you have a virus and another approach to use it as a therapy,” he says, “but having the same particle, the same approach, for both your detection and therapy.”
…
I wish Webster good luck. As for the rest us, let’s wash our hands and keep calm.


![The Endless Column, Târgu Jiu, România [downlaoded from http://terpconnect.umd.edu/~ckopetz/brancusi.htm]](http://www.frogheart.ca/wp-content/uploads/2013/12/EndlessColumn-200x300.jpg)