I have two pieces of research with the only common element being water. First, there’s a May 9, 2014 news release on EurekAlert issued by the Politecnico di Torino (Italy; rough translation: Turin Polytechnic),
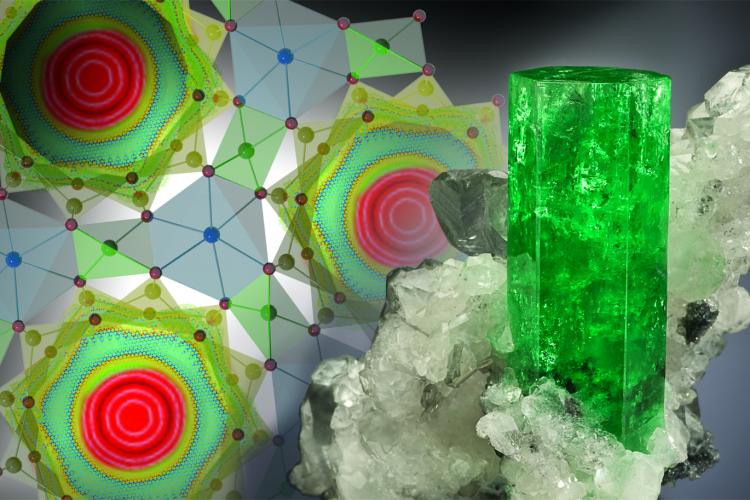
Swimming in a honey pool. That’s the sensation a water molecule should “feel” while approaching a solid surface within a nanometer (i.e. less than a ten-thousandth of hair diameter). The reduction in water mobility in the very close proximity of surfaces at the nanoscale is the well-known phenomenon of “nanoconfinement”, and it is due to both electrostatic and van der Waals attractive forces ruling matter interactions at that scale.
In this context, scientists from Politecnico di Torino and Houston Methodist Research Institute have taken a further step forward, by formulating a quantitative model and a physical interpretation able of predicting the nanoconfinement effect in a rather general framework. In particular, geometric and chemical characteristics as well as physical conditions of diverse nanoconfining surfaces (e.g. proteins, carbon nanotubes, silica nanopores or iron oxide nanoparticles) have been quantitatively related to mobility reduction and “supercooling” conditions of water, namely the persistence of water in a liquid state at temperatures far below 0°C, when close to a solid surface.
This result has been achieved after two years of in silico (i.e. computer-based) and in vitro (i.e. experiment-driven) activities by Eliodoro Chiavazzo, Matteo Fasano, Pietro Asinari (Multi-Scale Modelling Lab, Department of Energy at Politecnico di Torino) and Paolo Decuzzi (Center for the Rational Design of Multifunctional Nanoconstructs at Houston Methodist Research Institute).
I love the image of swimming in a ‘honey pool’ and while developing a schema for predicting a nanoconfinement effect may not seem all that exciting to an outsider the applications are varied according to the news release,
This study may soon find applications in the optimization and rational design of a broad variety of novel technologies ranging from applied physics (e.g. “nanofluids”, suspensions made out of water and nanoparticles for enhancing heat transfer) to sustainable energy (e.g. thermal storage based on nanoconfined water within sorbent materials); from detection and removal of pollutant from water (e.g. molecular sieves) to nanomedicine.
In fact this work is finding an immediate application in the field of medicine as pertaining to magnetic resonance imaging (MRI), from the news release,
The latter is the field where the research has indeed found a first important application. Every year, almost sixty millions of Magnetic Resonance Imaging (MRI) scans are performed, with diagnostic purposes. In the past decade, MRI technology benefitted from various significant scientific advances, which allowed more precise and sharper images of pathological tissues. Among other, contrast agents (i.e. substances used for improving contrast of structures or fluids within the body) importantly contributed in enhancing MRI performances.
This research activity has been able to explain and predict the increase in MRI performances due to nanoconfined contrast agents, which are currently under development at the Houston Methodist Research Institute. Hence, the discovery paves the way to further increase in the quality of MRI images, in order to possibly improve chances of earlier and more accurate detection of diseases in millions of patients, every year.
Here’s a link to and a citation for the research paper,
Scaling behaviour for the water transport in nanoconfined geometries by Eliodoro Chiavazzo, Matteo Fasano, Pietro Asinari & Paolo Decuzzi. Nature Communications 5 Article number: 4565 doi:10.1038/ncomms4565 Published 03 April 2014
This is an open access paper and, unusually, I am excerpting the Abstract as I find it helps to further explain this work (although the more technical aspects are lost on me),
The transport of water in nanoconfined geometries is different from bulk phase and has tremendous implications in nanotechnology and biotechnology. Here molecular dynamics is used to compute the self-diffusion coefficient D of water within nanopores, around nanoparticles, carbon nanotubes and proteins. For almost 60 different cases, D is found to scale linearly with the sole parameter θ as D(θ)=DB[1+(DC/DB−1)θ], with DB and DC the bulk and totally confined diffusion of water, respectively. The parameter θ is primarily influenced by geometry and represents the ratio between the confined and total water volumes. The D(θ) relationship is interpreted within the thermodynamics of supercooled water. As an example, such relationship is shown to accurately predict the relaxometric response of contrast agents for magnetic resonance imaging. The D(θ) relationship can help in interpreting the transport of water molecules under nanoconfined conditions and tailoring nanostructures with precise modulation of water mobility.
The second piece of ‘water’ research was featured in a May 13, 2014 news item on Nanowerk,
A simple new technique to form interlocking beads of water in ambient conditions could prove valuable for applications in biological sensing, membrane research and harvesting water from fog.
Researchers at the Department of Energy’s Oak Ridge National Laboratory have developed a method to create air-stable water droplet networks known as droplet interface bilayers. These interconnected water droplets have many roles in biological research because their interfaces simulate cell membranes. Cumbersome fabrication methods, however, have limited their use.
A May 13, 2014 Oak Ridge National Laboratory (ORNL) news release, which originated the news item, provides more details,
“The way they’ve been made since their inception is that two water droplets are formed in an oil bath then brought together while they’re submerged in oil,” said ORNL’s Pat Collier, who led the team’s study published in the Proceedings of the National Academy of Sciences. “Otherwise they would just pop like soap bubbles.”
Instead of injecting water droplets into an oil bath, the ORNL research team experimented with placing the droplets on a superhydrophobic surface infused with a coating of oil. The droplets aligned side by side without merging.
To the researchers’ surprise, they were also able to form non-coalescing water droplet networks without including lipids in the water solution. Scientists typically incorporate phospholipids into the water mixture, which leads to the formation of an interlocking lipid bilayer between the water droplets.
“When you have those lipids at the interfaces of the water drops, it’s well known that they won’t coalesce because the interfaces join together and form a stable bilayer,” ORNL coauthor Jonathan Boreyko said. “So our surprise was that even without lipids in the system, the pure water droplets on an oil-infused surface in air still don’t coalesce together.”
The team’s research revealed how the unexpected effect is caused by a thin oil film that is squeezed between the pure water droplets as they come together, preventing the droplets from merging into one. Watch a video of the process on ORNL’s YouTube channel.
With or without the addition of lipids, the team’s technique offers new insight for a host of applications. Controlling the behavior of pure water droplets on oil-infused surfaces is key to developing dew- or fog-harvesting technology as well as more efficient condensers, for instance.
“Our finding of this non-coalescence phenomenon will shed light on these droplet-droplet interactions that can occur on oil-infused systems,” Boreyko said.
The ability to create membrane-like water droplet networks by adding lipids leads to a different set of functional applications, Collier noted.
“These bilayers can be used in anything from synthetic biology to creating circuits to bio-sensing applications,” he said. “For example, we could make a bio-battery or a signaling network by stringing some of these droplets together. Or, we could use it to sense the presence of airborne molecules.”
The team’s study also demonstrated ways to control the performance and lifetime of the water droplets by manipulating oil viscosity and temperature and humidity levels.
Here’s another link to the paper and a citation,
Air-stable droplet interface bilayers on oil-infused surfaces by Jonathan B. Boreyko, Georgios Polizos, Panos G. Datskos, Stephen A. Sarles, and C. Patrick Collier. PNAS 2014 ; published ahead of print May 12, 2014, doi: 10.1073/pnas.1400381111
This paper is behind a paywall.