A July 30, 2020 news item on ScienceDaily announces news about more accurate health monitoring with electronics applied directly to your skin,
A team of researchers led by Cunjiang Yu, Bill D. Cook Associate Professor of Mechanical Engineering at the University of Houston, has developed a new form of electronics known as “drawn-on-skin electronics,” allowing multifunctional sensors and circuits to be drawn on the skin with an ink pen.
The advance, the researchers report in Nature Communications, allows for the collection of more precise, motion artifact-free health data, solving the long-standing problem of collecting precise biological data through a wearable device when the subject is in motion.
The imprecision may not be important when your FitBit registers 4,000 steps instead of 4,200, but sensors designed to check heart function, temperature and other physical signals must be accurate if they are to be used for diagnostics and treatment.
…
A July 30, 2020 University of Houston news release (also on EurekAlert) by Jeannie Kever, which originated the news item, goes on to explain why you might want to have electronics ‘drawn on your skin’,
The drawn-on-skin electronics are able to seamlessly collect data, regardless of the wearer’s movements.
They also offer other advantages, including simple fabrication techniques that don’t require dedicated equipment.
“It is applied like you would use a pen to write on a piece of paper,” said Yu. “We prepare several electronic materials and then use pens to dispense them. Coming out, it is liquid. But like ink on paper, it dries very quickly.”
Wearable bioelectronics – in the form of soft, flexible patches attached to the skin – have become an important way to monitor, prevent and treat illness and injury by tracking physiological information from the wearer. But even the most flexible wearables are limited by motion artifacts, or the difficulty that arises in collecting data when the sensor doesn’t move precisely with the skin.
The drawn-on-skin electronics can be customized to collect different types of information, and Yu said it is expected to be especially useful in situations where it’s not possible to access sophisticated equipment, including on a battleground.
The electronics are able to track muscle signals, heart rate, temperature and skin hydration, among other physical data, he said. The researchers also reported that the drawn-on-skin electronics have demonstrated the ability to accelerate healing of wounds.
In addition to Yu, researchers involved in the project include Faheem Ershad, Anish Thukral, Phillip Comeaux, Yuntao Lu, Hyunseok Shim, Kyoseung Sim, Nam-In Kim, Zhoulyu Rao, Ross Guevara, Luis Contreras, Fengjiao Pan, Yongcao Zhang, Ying-Shi Guan, Pinyi Yang, Xu Wang and Peng Wang, all from the University of Houston, and Jiping Yue and Xiaoyang Wu from the University of Chicago.
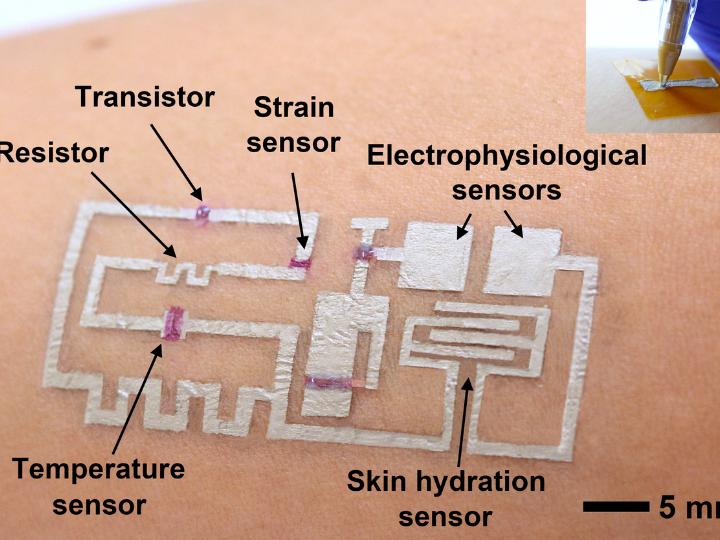
The drawn-on-skin electronics are actually comprised of three inks, serving as a conductor, semiconductor and dielectric.
“Electronic inks, including conductors, semiconductors, and dielectrics, are drawn on-demand in a freeform manner to develop devices, such as transistors, strain sensors, temperature sensors, heaters, skin hydration sensors, and electrophysiological sensors,” the researchers wrote.
This research is supported by the Office of Naval Research and National Institutes of Health.
Here’s a link to and a citation for the paper,
Ultra-conformal drawn-on-skin electronics for multifunctional motion artifact-free sensing and point-of-care treatment by Faheem Ershad, Anish Thukral, Jiping Yue, Phillip Comeaux, Yuntao Lu, Hyunseok Shim, Kyoseung Sim, Nam-In Kim, Zhoulyu Rao, Ross Guevara, Luis Contreras, Fengjiao Pan, Yongcao Zhang, Ying-Shi Guan, Pinyi Yang, Xu Wang, Peng Wang, Xiaoyang Wu & Cunjiang Yu. Nature Communications volume 11, Article number: 3823 (2020) DOI: https://doi.org/10.1038/s41467-020-17619-1
This paper is open access.
![Bacteriorhodopsin is the only protein of purple membranes, which contains few different lipids. [downloaded from the University of Bari: http://www.biologia.uniba.it/fisiologia/corcelli/en/ric2.html]](http://www.frogheart.ca/wp-content/uploads/2013/07/purpleBacteriorhodopsin-300x225.jpg)