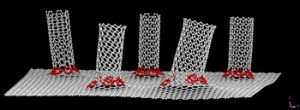
An armchair carbon nanotube is amongst the most desirable of carbon nanotubes. You’ll have to look carefully to see the resemblance to an armchair,
![Armchair carbon nanotubes, so named for the arrangement of atoms that make their ends look like armchairs, are the most desirable among nanotube researchers for their superior electrical properties. Image by Erik Hároz [downloaded from http://news.rice.edu/2013/02/05/essential-armchair-reading-for-nanotube-researchers-2/]](http://www.frogheart.ca/wp-content/uploads/2013/02/ArmchairCarbonNanotube-300x251.jpeg)
Armchair carbon nanotubes, so named for the arrangement of atoms that make their ends look like armchairs, are the most desirable among nanotube researchers for their superior electrical properties. Image by Erik Hároz [downloaded from http://news.rice.edu/2013/02/05/essential-armchair-reading-for-nanotube-researchers-2/]
The
Feb. 6, 2013 news item on phys.org about the armchair carbon nanotubes notes that this latest research is an early outcome from a
recently announced (Oct. 2012) partnership between Rice University and the US National Institute of Standards and Technology (NIST). Trom the news item (Note: Links have been removed),
The first fruits of a cooperative venture between scientists at Rice University and the National Institute of Standards and Technology (NIST) have appeared in a paper that brings together a wealth of information for those who wish to use the unique properties of metallic carbon nanotubes.
The feature article published recently in the Royal Society of Chemistry journal Nanoscale gathers research about the separation and fundamental characteristics of armchair carbon nanotubes, which have been of particular interest to researchers trying to tune their electronic and optical properties.
The Rice University Feb. 5, 2013 news release by Mike Williams, which originated the news item, describe s the process the scientists undertook,
This paper, said Rice physicist Junichiro Kono, provides scientists a valuable resource for detailed information about metallic carbon nanotubes, especially armchair nanotubes. “Basically, we summarized all our recent findings as well as all information we could find in the literature about metallic nanotubes, along with detailed accounts of preparation methods for metal-enriched nanotube samples, to show the community just how much we now understand about these one-dimensional metals,” he said.
As part of the lengthy work, the team compiled and published tables of essential statistics, including optical properties, for a variety of metallic nanotubes. “We provide fundamental theoretical backgrounds and then show very detailed experimental results on unique properties of metallic nanotubes,” Kono said. “This paper summarizes what kind of aspects are understood, and what is not, about fundamental optical processes in nanotubes and will make it easier for researchers to identify their spectroscopic features and transition energies.”
For this of us who are less well versed on armchair carbon nanotubes and their electronic and optical properties, the news releases provides some information (Note: Links have been removed),
Nanotubes come in many flavors, depending on their chirality. Chirality is a characteristic akin to the angles at which a flat sheet of paper might align when wrapped into a tube. Cut the tube in half and the atoms at the open edge would line up in the shape of an armchair, a zigzag or some variant. Even though their raw material is identical – chicken-wire-like hexagons of carbon – the chirality makes all the difference in how nanotubes transmit electricity.
Armchairs are the most coveted because they have no band gap; electrons flow through without resistance. Cables made with armchair nanotubes have the potential to move electricity over great distances with virtually no loss. That makes them the gold standard as the basic element of armchair quantum wire. The ongoing development of this very strong, lightweight, high-capacity cable could improve further the record properties of multifunctional carbon nanotube fibers that are being developed by the group of Rice Professor Matteo Pasquali.
For the project-specific work the scientists performed (Note: Links have been removed),
The new work led by Kono and Robert Hauge, a distinguished faculty fellow in chemistry at Rice, along with scientists at NIST and Los Alamos National Laboratory, looks beyond the armchair’s established electrical properties to further detail their potential for electronic, sensing, optical and photonic devices.
“Of course, to get there, we need really good samples,” Kono said. “Many applications will rely on our ability to separate carbon nanotubes and then assemble macroscopically ordered structures consisting of single-chirality nanotubes. Nobody can do that at this point.”
When a batch of nanotubes comes out of a furnace, it’s a jumble of types. That makes detailed analysis of their characteristics — let alone their practical use — a challenge.
But techniques developed in recent years at Rice and by NIST scientist Ming Zheng to purify metallic nanotubes are beginning to change that. Rice graduate student Erik Hároz said recent experiments established “unambiguous evidence” that a process he and Kono are using called density gradient ultracentrifugation can enrich ensemble samples of armchairs. Taking things further, Zheng’s method of DNA-based ion-exchange chromatography provides very small samples of ultrapure armchair nanotubes of a single chirality.
You can read more about the work at phys.org or at Rice University using the links already provided. For those who’d like to read the research,
Fundamental optical processes in armchair carbon nanotubes by Erik H. Hároz , Juan G. Duque, Xiaomin Tu , Ming Zheng , Angela R. Hight Walker , Robert H. Hauge , Stephen K. Doorn and Junichiro Kono. Nanoscale, 2013,5, 1411-1439 DOI: 10.1039/C2NR32769D First published on the web 04 Jan 2013
This article is behind a paywall of sorts. RSC (Royal Society of Chemistry) Publishing (which publishes Nanoscale) has an open access policy but there are various options, from the RSC Publishing’s Open Access Policy webpage,
RSC Open Access statement
Open Access is the term given to making electronic versions of articles accessible to readers, without any subscription or ‘access side’ fees.
RSC supports Open Access models which seek to ensure that scholarly publishing activities operate in a long term sustainable way.
- Our fundamental goal is to advance the chemical sciences, through the effective dissemination of high quality research content
- We seek to maximise the dissemination of the research that we publish
- We support any and all sustainable and fair models of access. We believe that the integrity and archiving of scholarly content must be maintained throughout
- We support ‘Gold’* Open Access and encourage funding to be made available to support authors during any transition from reader to author side payments
- We support the author’s ability to choose where they publish their work to the benefit of the advancement of science. We do not wish authors to be discriminated against if they are unable to pay author-side fees
- We seek to work closely with other parties, including funders and government agencies, to achieve the above goals
RSC Publishing provides authors with the option to make their article Open Access, through payment of a fee on acceptance. Authors following the traditional route still have deposition options – details are on the ‘Deposition and Licence to Publish’ page of the website.
*There are several types of Open Access:
- Gold Open Access: Publication costs are covered by an ‘Article Processing Fees’ being paid by authors upon acceptance. The final ‘article of record’ is made available to all, immediately, without any barriers to access
- Green Open Access: A version of the paper (often the author’s manuscript) is made available via a subject or institutional repository. An embargo period is often involved, typically 6-24 months. No payment is made, and publishers should strive to recoup their investment through traditional sales during the embargo period
- Delayed Open Access: The final version of the paper is made available by the publisher after an embargo period (e.g. publisher deposit the paper in PubMed after 12 months)
It would seem the option for this article is ‘Delayed Open Access’.
![Armchair carbon nanotubes, so named for the arrangement of atoms that make their ends look like armchairs, are the most desirable among nanotube researchers for their superior electrical properties. Image by Erik Hároz [downloaded from http://news.rice.edu/2013/02/05/essential-armchair-reading-for-nanotube-researchers-2/]](http://www.frogheart.ca/wp-content/uploads/2013/02/ArmchairCarbonNanotube-300x251.jpeg)