A friend sent me a link to this interview with Iftach Yacoby of Tel Aviv University talking about some new research into plants and electricity. From a June 8, 2020 article by Omer Kabir for Calcalist (CTech) on the Algemeiner website,
For years, scientists have been trying to understand the evolutionary capabilities of plants to produce energy and have had only partial success. But a recent Tel Aviv University [TAU] study seems to make the impossible possible, proving that any plant can be transformed into an electrical source, producing a variety of materials that can revolutionize the global economy — from using hydrogen as fuel to clean ammonia to replace the pollutants in the agriculture industry.
“People are unaware that their plant pots have an electric current for everything,” Iftach Yacoby, head of the Laboratory of Renewable Energy Studies at Tel Aviv University’s Faculty of Life Sciences said in a recent interview with Calcalist.
“Our study opens the door to a new field of agriculture, equivalent to wheat or corn production for food security — generating energy,” he said. However, Yacoby makes it clear that it will take at least a decade before the research findings can be transferred to the commercial level.
…
At the heart of the research is the understanding that plants have particularly efficient capacities when it comes to electricity generation. “Anything green that is not dollars, but rather leaves, grass, and seaweed for example, contains solar panels that are completely identical to the panels the entire country is now building,” Yacoby explained. “They know how to take in solar radiation and make electrons flow out of it. That’s the essence of photosynthesis. Most people think of oxygen and food production, but the most basic phase of photosynthesis is the same as silicon panels in the Negev and on rooftops — taking in sunlight and generating electric current.”
… “At home, an electric current can be wired to many devices. Just plug the device into a power outlet. But when you want to do it in plants, it’s about the order of nanometers. We have no idea where to plug the plugs. That’s what we did in this study. In plant cells, we found they can be used as a socket for anything, at just a nanometer size. We have an enzyme, which is equivalent to a biological machine that can produce hydrogen. We took this enzyme, put it together so that it sits in the socket in the plant cell, which was previously only hypothetical. When he started to produce hydrogen, we proved that we had a socket for everything, though nanotermically-sized. Now we can take any plant or kelp and engineer it so that their electrical outlet can be used for production purposes,” Yacoby explained.
“If you attach an enzyme that produces hydrogen you get hydrogen, it’s the cleanest fuel that can be,” he said. “There are already electric cars and bicycles with a range of 150 km that travel on hydrogen. There are many types of enzymes in nature that produce valuable substances, such as ammonia needed for the fertilizer industry and today is still produced by a very toxic and harmful method that consumes a lot of energy. We can provide a plant-based alternative for the production of materials that are made in chemical manufacturing facilities. It’s an electric platform inside a living plant cell.”
…
You might find it helpful to read Kabir’s article in its entirety before moving on to the news release about the work. The work was conducted with researchers from Arizona State University (ASU;US) and a researcher from Yogi Vemana University (India), as well as, Yacoby. There’s a May 7, 2020 ASU news release (also on EurekAlert but published on May 6, 2020) detailing the work,
Hydrogen is an essential commodity with over 60 million tons produced globally every year. However over 95 percent of it is made by steam reformation of fossil fuels, a process that is energy intensive and produces carbon dioxide. If we could replace even a part of that with algal biohydrogen that is made via light and water, it would have a substantial impact.
This is essentially what has just been achieved in the lab of Kevin Redding, professor in the School of Molecular Sciences and director of the Center for Bioenergy and Photosynthesis. Their research, entitled Rewiring photosynthesis: a Photosystem I -hydrogenase chimera that makes hydrogen in vivo was published very recently in the high impact journal Energy and Environmental Science.
“What we have done is to show that it is possible to intercept the high energy electrons from photosynthesis and use them to drive alternate chemistry, in a living cell” explained Redding. “We have used hydrogen production here as an example.”
“Kevin Redding and his group have made a true breakthrough in re-engineering the Photosystem I complex,” explained Ian Gould, interim director of the School of Molecular Sciences, which is part of The College of Liberal Arts and Sciences. “They didn’t just find a way to redirect a complex protein structure that nature designed for one purpose to perform a different, but equally critical process, but they found the best way to do it at the molecular level.”
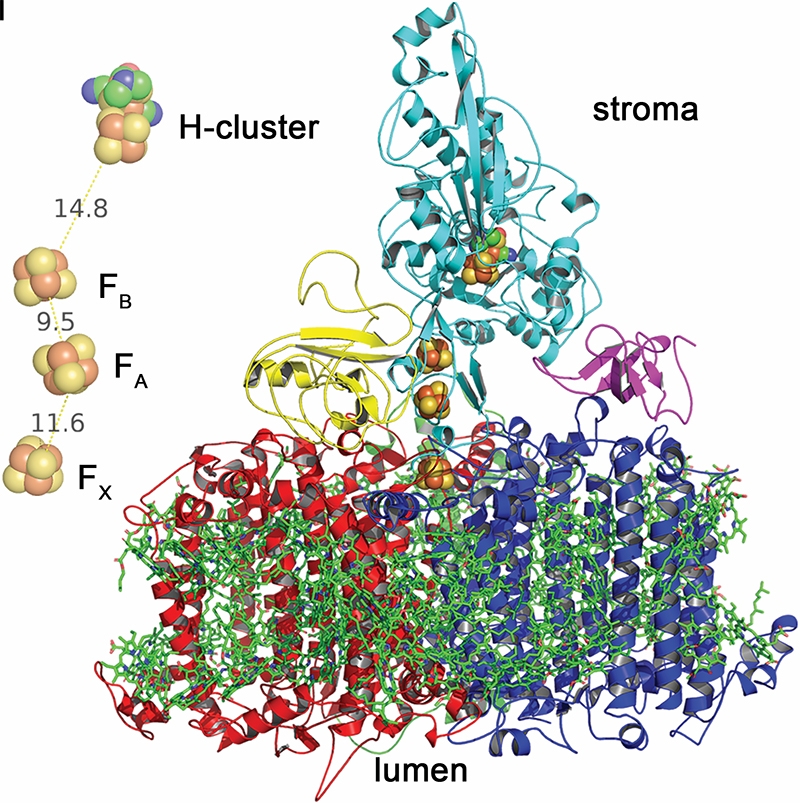
It is common knowledge that plants and algae, as well as cyanobacteria, use photosynthesis to produce oxygen and “fuels,” the latter being oxidizable substances like carbohydrates and hydrogen. There are two pigment-protein complexes that orchestrate the primary reactions of light in oxygenic photosynthesis: Photosystem I (PSI) and Photosystem II (PSII).
Algae (in this work the single-celled green alga Chlamydomonas reinhardtii, or ‘Chlamy’ for short) possess an enzyme called hydrogenase that uses electrons it gets from the protein ferredoxin, which is normally used to ferry electrons from PSI to various destinations. A problem is that the algal hydrogenase is rapidly and irreversibly inactivated by oxygen that is constantly produced by PSII.
In this study, doctoral student and first author Andrey Kanygin has created a genetic chimera of PSI and the hydrogenase such that they co-assemble and are active in vivo. This new assembly redirects electrons away from carbon dioxide fixation to the production of biohydrogen.
“We thought that some radically different approaches needed to be taken — thus, our crazy idea of hooking up the hydrogenase enzyme directly to Photosystem I in order to divert a large fraction of the electrons from water splitting (by Photosystem II) to make molecular hydrogen,” explained Redding.
Cells expressing the new photosystem (PSI-hydrogenase) make hydrogen at high rates in a light dependent fashion, for several days.
This important result will also be featured in an upcoming article in Chemistry World – a monthly chemistry news magazine published by the Royal Society of Chemistry. The magazine addresses current developments in the world of chemistry including research, international business news and government policy as it affects the chemical science community.
The NSF grant funding this research is part of the U.S.-Israel Binational Science Foundation (BSF). In this arrangement, a U.S. scientist and Israeli scientist join forces to form a joint project. The U.S. partner submits a grant on the joint project to the NSF, and the Israeli partner submits the same grant to the ISF (Israel Science Foundation). Both agencies must agree to fund the project in order to obtain the BSF funding. Professor Iftach Yacoby of Tel Aviv University, Redding’s partner on the BSF project, is a young scientist who first started at TAU about eight years ago and has focused on different ways to increase algal biohydrogen production.
In summary, re-engineering the fundamental processes of photosynthetic microorganisms offers a cheap and renewable platform for creating bio-factories capable of driving difficult electron reactions, powered only by the sun and using water as the electron source.
Here’s a link to and a citation for the paper,
Rewiring photosynthesis: a photosystem I-hydrogenase chimera that makes H2in vivo† by Andrey Kanygin, Yuval Milrad, Chandrasekhar Thummala, Kiera Reifschneider, Patricia Baker, Pini Marco, Iftach Yacoby and Kevin E. Redding. Energy Environ. Sci., 2020, Advance DOI: https://doi.org/10.1039/C9EE03859K First published: 17 Apr 2020
In order to gain access to the paper, you must have or sign up for a free account.
This image was used to illustrate the research,