Researchers at the University of Maryland (US) have found a way to make their wood transparent by using sunlight. From a February 2, 2021 news article by Bob Yirka on phys.org (Note: Links have been removed),
A team of researchers at the University of Maryland, has found a new way to make wood transparent. In their paper published in the journal Science Advances, the group describes their process and why they believe it is better than the old process.
…
The conventional method for making wood transparent involves using chemicals to remove the lignin—a process that takes a long time, produces a lot of liquid waste and results in weaker wood. In this new effort, the researchers have found a way to make wood transparent without having to remove the lignin.
The process involved changing the lignin rather than removing it. The researchers removed lignin molecules that are involved in producing wood color. First, they applied hydrogen peroxide to the wood surface and then exposed the treated wood to UV light (or natural sunlight). The wood was then soaked in ethanol to further clean it. Next, they filled in the pores with clear epoxy to make the wood smooth.
…
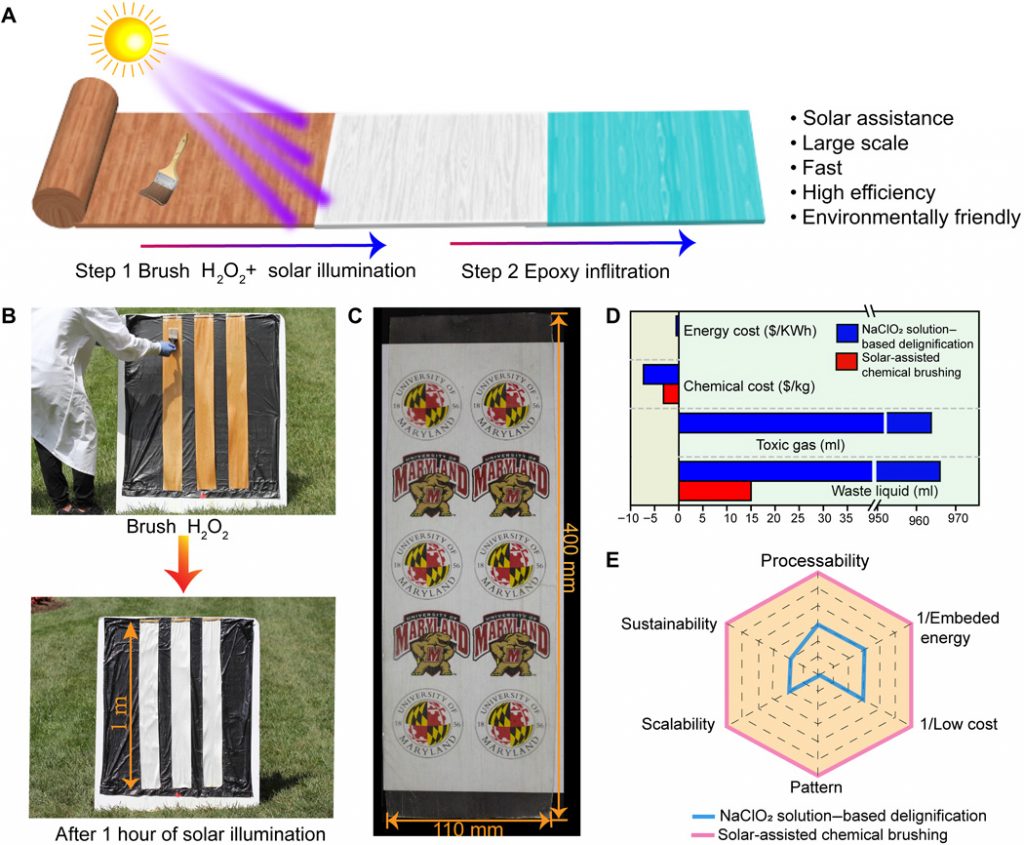
Bob McDonald in a February 5, 2021 posting on his Canadian Broadcasting Corporation (CBC) Quirks & Quarks blog provides a more detailed description of the new ‘solar-based transparency process’,
Early attempts to make transparent wood involved removing the lignin, but this involved hazardous chemicals, high temperatures and a lot of time, making the product expensive and somewhat brittle. The new technique is so cheap and easy it could literally be done in a backyard.
Starting with planks of wood a metre long and one millimetre thick, the scientists simply brushed on a solution of hydrogen peroxide using an ordinary paint brush. When left in the sun, or under a UV lamp for an hour or so, the peroxide bleached out the brown chromophores but left the lignin intact, so the wood turned white.
Next, they infused the wood with a tough transparent epoxy designed for marine use, which filled in the spaces and pores in the wood and then hardened. This made the white wood transparent.
…
As window material, it would be much more resistant to accidental breakage. The clear wood is lighter than glass, with better insulating properties, which is important because windows are a major source of heat loss in buildings. It also might take less energy to manufacture clear wood because there are no high temperatures involved.
…
Many different types of wood, from balsa to oak, can be made transparent, and it doesn’t matter if it is cut along the grain or against it. If the transparent wood is made a little thicker, it would be strong enough to become part of the structure of a building, so there could be entire transparent wooden walls.
…
Adele Peters in her February 2, 2021 article for Fast Company describes the work in Maryland and includes some information about other innovative and possibly sustainable uses of wood (Note: Links have been removed),
It’s [transparent wood] just one of a number of ways scientists and engineers are rethinking how we can use this renewable resource in construction. Skyscrapers made entirely out of wood are gaining popularity in cities around the world. And scientists recently discovered a technique to grow wood in a lab, opening up the possibility of using wood without having to chop down a forest.
There were three previous posts here about this work at the University of Maryland,
University of Maryland looks into transparent wood May 11, 2016 posting
Transparent wood more efficient than glass in windows? Sept, 8, 2016 posting
Glass-like wood windows protect against UV rays and insulate heat October 21, 2020 posting
I have this posting, which is also from 2016 but features work in Sweden,
Transparent wood instead of glass for window panes? April 1, 2016 posting
Getting back to the latest work from the University of Maryland, here’s a link to and a citation for the paper,
Solar-assisted fabrication of large-scale, patternable transparent wood by Qinqin Xia, Chaoji Chen, Tian Li, Shuaiming He, Jinlong Gao, Xizheng Wang and Liangbing Hu. Science Advances Vol. 7, no. 5, eabd7342 DOI: 10.1126/sciadv.abd7342 Published: 27 Jan 2021
This paper is open access.
One last item, Liangbing Hu has founded a company InventWood for commercializing the work he and his colleagues have done at the University of Maryland.



![[downloaded from http://pubs.acs.org/doi/abs/10.1021/am505677x]](http://www.frogheart.ca/wp-content/uploads/2014/11/SumlightBPaDegradation-300x127.gif)