According to an April 1, 2016 news item on Nanowerk, the US National Nanotechnology (NNI) has released its 2017 budget supplement,
The President’s Budget for Fiscal Year 2017 provides $1.4 billion for the National Nanotechnology Initiative (NNI), affirming the important role that nanotechnology continues to play in the Administration’s innovation agenda. NNI
Cumulatively totaling nearly $24 billion since the inception of the NNI in 2001, the President’s 2017 Budget supports nanoscale science, engineering, and technology R&D at 11 agencies.Another 9 agencies have nanotechnology-related mission interests or regulatory responsibilities.
An April 1, 2016 NNI news release, which originated the news item, affirms the Obama administration’s commitment to the NNI and notes the supplement serves as an annual report amongst other functions,
Throughout its two terms, the Obama Administration has maintained strong fiscal support for the NNI and has implemented new programs and activities to engage the broader nanotechnology community to support the NNI’s vision that the ability to understand and control matter at the nanoscale will lead to new innovations that will improve our quality of life and benefit society.
This Budget Supplement documents progress of these participating agencies in addressing the goals and objectives of the NNI. It also serves as the Annual Report for the NNI called for under the provisions of the 21st Century Nanotechnology Research and Development Act of 2003 (Public Law 108-153, 15 USC §7501). The report also addresses the requirement for Department of Defense reporting on its nanotechnology investments, per 10 USC §2358.
For additional details and to view the full document, visit www.nano.gov/2017BudgetSupplement.
I don’t seem to have posted about the 2016 NNI budget allotment but 2017’s $1.4B represents a drop of $100M since 2015’s $1.5 allotment.
The 2017 NNI budget supplement describes the NNI’s main focus,
Over the past year, the NNI participating agencies, the White House Office of Science and Technology Policy (OSTP), and the National Nanotechnology Coordination Office (NNCO) have been charting the future directions of the NNI, including putting greater focus on promoting commercialization and increasing education and outreach efforts to the broader nanotechnology community. As part of this effort, and in keeping with recommendations from the 2014 review of the NNI by the President’s Council of Advisors for Science and Technology, the NNI has been working to establish Nanotechnology-Inspired Grand Challenges, ambitious but achievable goals that will harness nanotechnology to solve National or global problems and that have the potential to capture the public’s imagination. Based upon inputs from NNI agencies and the broader community, the first Nanotechnology-Inspired Grand Challenge (for future computing) was announced by OSTP on October 20, 2015, calling for a collaborative effort to “create a new type of computer that can proactively interpret and learn from data, solve unfamiliar problems using what it has learned, and operate with the energy efficiency of the human brain.” This Grand Challenge has generated broad interest within the nanotechnology community—not only NNI agencies, but also industry, technical societies, and private foundations—and planning is underway to address how the agencies and the community will work together to achieve this goal. Topics for additional Nanotechnology-Inspired Grand Challenges are under review.
Interestingly, it also offers an explanation of the images on its cover (Note: Links have been removed),
About the cover
Each year’s National Nanotechnology Initiative Supplement to the President’s Budget features cover images illustrating recent developments in nanotechnology stemming from NNI activities that have the potential to make major contributions to National priorities. The text below explains the significance of each of the featured images on this year’s cover.
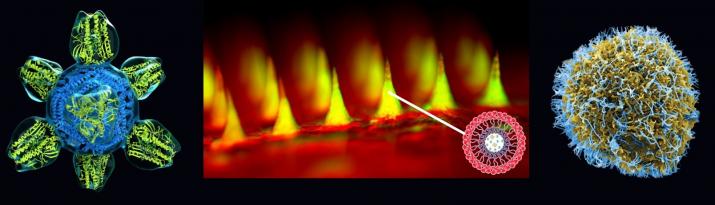
Front cover featured images (above): Images illustrating three novel nanomedicine applications. Center: microneedle array for glucose-responsive insulin delivery imaged using fluorescence microscopy. This “smart insulin patch” is based on painless microneedles loaded with hypoxia-sensitive vesicles ~100 nm in diameter that release insulin in response to high glucose levels. Dr. Zhen Gu and colleagues at the University of North Carolina (UNC) at Chapel Hill and North Carolina State University have demonstrated that this patch effectively regulates the blood glucose of type 1 diabetic mice with faster response than current pH-sensitive formulations. The inset image on the lower right shows the structure of the nanovesicles; each microneedle contains more than 100 million of these vesicles. The research was supported by the American Diabetes Association, the State of North Carolina, the National Institutes of Health (NIH), and the National Science Foundation (NSF). Left: colorized rendering of a candidate universal flu vaccine nanoparticle. The vaccine molecule, developed at the NIH Vaccine Research Center, displays only the conserved part of the viral spike and stimulates the production of antibodies to fight against the ever-changing flu virus. The vaccine is engineered from a ~13 nm ferritin core (blue) combined with a 7 nm influenza antigen (green). Image credit: NIH National Institute of Allergy and Infectious Diseases (NIAID). Right: colorized scanning electron micrograph of Ebola virus particles on an infected VERO E6 cell. Blue represents individual Ebola virus particles. The image was produced by John Bernbaum and Jiro Wada at NIAID. When the Ebola outbreak struck in 2014, the Food and Drug Administration authorized emergency use of lateral flow immunoassays for Ebola detection that use gold nanoparticles for visual interpretation of the tests.
Back cover featured images (above): Images illustrating examples of NNI educational outreach activities. Center: Comic from the NSF/NNI competition Generation Nano: Small Science Superheroes. Illustration by Amina Khan, NSF. Left of Center: Polymer Nanocone Array (biomimetic of antimicrobial insect surface) by Kyle Nowlin, UNC-Greensboro, winner from the first cycle of the NNI’s student image contest, EnvisioNano. Right of Center: Gelatin Nanoparticles in Brain (nasal delivery of stroke medication to the brain) by Elizabeth Sawicki, University of Illinois at Urbana-Champaign, winner from the second cycle of EnvisioNano. Outside right: still photo from the video Chlorination-less (water treatment method using reusable nanodiamond powder) by Abelardo Colon and Jennifer Gill, University of Puerto Rico at Rio Piedras, the winning video from the NNI’s Student Video Contest. Outside left: Society of Emerging NanoTechnologies (SENT) student group at the University of Central Florida, one of the initial nodes in the developing U.S. Nano and Emerging Technologies Student Network; photo by Alexis Vilaboy.