I am fascinated by research that focuses on boundaries as does this work from Rice University (Texas, US) and the University of Bremen (Germany) but first a general description of the research from a Dec. 6, 2013 news item on Nanowerk (Note: A link has been removed),
Scientists from Rice University and the University of Bremen’s Center for Marine Environmental Sciences (MARUM) in Germany have combined cutting-edge experimental techniques and computer simulations to find a new way of predicting how water dissolves crystalline structures like those found in natural stone and cement.
In a new study featured on the cover of the Nov. 28 issue of the Journal of Physical Chemistry C (“Kinetic Monte Carlo Simulations of Silicate Dissolution: Model Complexity and Parametrization”), the team found their method was more efficient at predicting the dissolution rates of crystalline structures in water than previous methods. The research could have wide-ranging impacts in diverse areas, including water quality and planning, environmental sustainability, corrosion resistance and cement construction.
The Dec. 5, 2013 Rice University news release, which originated the news item, explains the reasons for the research and delves into the subject of boundaries,
“We need to gain a better understanding of dissolution mechanisms to better predict the fate of certain materials, both in nature and in man-made systems,” said lead investigator Andreas Lüttge, a professor of mineralogy at MARUM and professor emeritus and research professor in Earth science at Rice. His team specializes in studying the thin boundary layer that forms between minerals and fluids.
Boundary layers are ubiquitous in nature; they occur when raindrops fall on stone, water seeps through soil and the ocean meets the sea floor. Scientists and engineers have long been interested in accurately explaining how crystalline materials, including many minerals and stones, interact with and are dissolved by water. Calculations about the rate of these dissolution processes are critical in many fields of science and engineering.
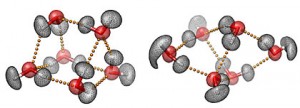
In the new study, Lüttge and lead author Inna Kurganskaya, a research associate in Earth science at Rice, studied dissolution processes using quartz, one of the most common minerals found in nature. Quartz, or silicon dioxide, is a type of silicate, the most abundant group of minerals in Earth’s crust.
At the boundary layer where quartz and water meet, multiple chemical reactions occur. Some of these happen simultaneously and others take place in succession. In the new study, the researchers sought to create a computerized model that could accurately simulate the complex chemistry at the boundary layer.
“The new model simulates the dissolution kinetics at the boundary layer with greater precision than earlier stochastic models operating at the same scale,” Kurganskaya said. “Existing simulations rely on rate constants assigned to a wide range of possible reactions, and as a result, the total material flux from the surface have an inherent variance range — a plus or minus factor that is always there.”
The team used new equipment to achieve increased imaging precision (from the news release),
One reason the team’s simulations more accurately represent real processes is that its models incorporate actual measurements from cutting-edge instruments and from high-tech materials, including glass ceramics and nanomaterials. With a special imaging technique called “vertical scanning interferometry,” which the group at MARUM and Rice helped to develop, the team scanned the crystal surfaces of both minerals and manufactured materials to generate topographic maps with a resolution of a just a few nanometers, or billionths of a meter.
“We found that dissolution rates that were predicted using rate constants were sometimes off by as much as two orders of magnitude,” Lüttge said.
The new method for more precisely predicting dissolution processes could revolutionize the way engineers and scientists make many calculations related to a myriad of things, including the stability of building materials, the longevity of materials used for radioactive waste storage and more, he said.
“Further work is needed to prove the broad utility of the method,” he said. “In the next phase of research, we plan to test our simulations on larger systems and over longer periods.”
One often sees funding information at the end of these types of news releases, which I don’t usually include here but I found this one a bit surprising (this is the first time I’ve seen research supported by a university that has no researchers involved in the work),
The research was supported by the Global Climate and Energy Project at Stanford University
The researchers offered this image to illustrate their work,
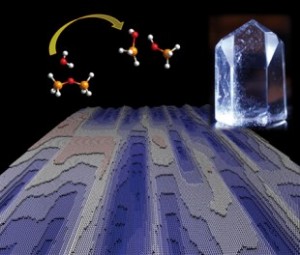
The dissolution process of a crystalline structure in water is shown: two bonded SiO4 — molecules dissolve (top left), a quartz crystal (top right) and the computer-simulated surface of a dissolving crystalline structure (below). CREDIT: MARUM & Rice University
For those who just can’t get enough information, here’s a link to and a citation for the paper,
Kinetic Monte Carlo Simulations of Silicate Dissolution: Model Complexity and Parametrization by Inna Kurganskaya and Andreas Luttge. J. Phys. Chem. C, 2013, 117 (47), pp 24894–24906 DOI: 10.1021/jp408845m Publication Date (Web): October 10, 2013
Copyright © 2013 American Chemical Society
This paper is behind a paywall.