This screams tattoo to me but it’s not,
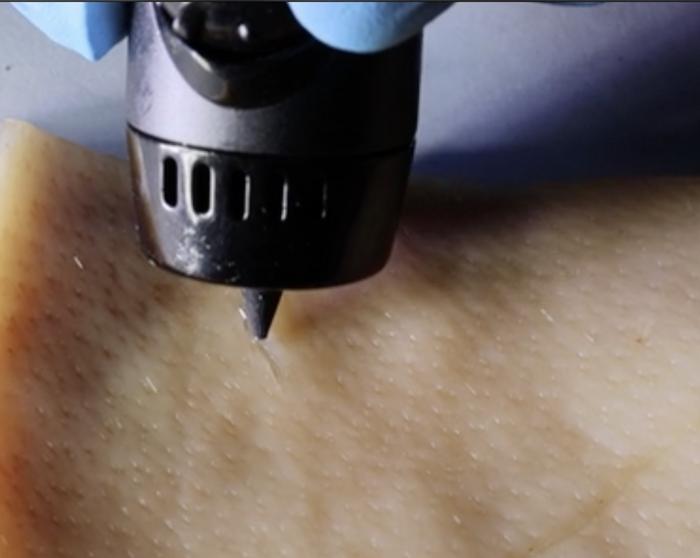
A June 1, 2023 American Chemical Society (ACS) news release (also on EurekAlert), announces a new approach to wound healing,
The body is pretty good at healing itself, though more severe wounds can require bandages or stitches. But researchers publishing in ACS Applied Materials & Interfaces have developed a wound-healing ink that can actively encourage the body to heal by exposing the cut to immune-system vesicles. The ink can be spread into a cut of any shape using a 3D-printing pen, and in mice, the technology nearly completely repaired wounds in just 12 days.
When the skin is cut or torn, the body’s natural “construction crew” kicks in to fix it back up — clearing out any bacterial invaders, regrowing broken blood vessels and eventually forming a scar. Many techniques used to heal wounds can’t do much beyond helping the body do its job better. Bandages or stitches are used to prevent further bleeding, while antibiotics work to prevent complications from infections. But by adding members of the construction crew to a wound-healing treatment or bandage, it could actually accelerate the natural healing process. Specifically, white blood cells or the extracellular vesicles (EVs) secreted from them play important roles in promoting blood vessel formation and reducing inflammation during healing. So, Dan Li, Xianguang Ding and Lianhui Wang wanted to incorporate these EVs into a hydrogel-based wound healing ink that could be painted into cuts of any shape.
The team developed a system called PAINT, or “portable bioactive ink for tissue healing,” using EVs secreted from macrophages combined with sodium alginate. These components were combined in a 3D-printing pen, where they mixed at the pen’s tip and formed a sturdy gel at the site of injury within three minutes. The EVs promoted blood vessel formation and reduced inflammatory markers in human epithelial cells, shifting them into the “proliferative,” or growth, phase of healing. PAINT was also tested on injured mice, where it promoted collagen fiber formation. Mice treated with PAINT had almost healed completely from a large wound after 12 days, compared to mice that didn’t receive the treatment, who were not nearly as far along in the healing process at this time point. The researchers say that this work could help heal a wide variety of cuts quickly and easily, without the need for complex procedures.
The authors acknowledge funding from the Leading-Edge Technology Programme of Jiangsu Natural Science Foundation, the Natural Science Foundation, the Natural Science Foundation of Jiangsu Province, the CAS [Chinese Academy of Sciences] Key Laboratory of Nano-Bio Interface, the Key Laboratory of Nanodevices and Applications, and the Postgraduate Research & Practice Innovation Program of Jiangsu Province.
The American Chemical Society (ACS) is a nonprofit organization chartered by the U.S. Congress. ACS’ mission is to advance the broader chemistry enterprise and its practitioners for the benefit of Earth and all its people. The Society is a global leader in promoting excellence in science education and providing access to chemistry-related information and research through its multiple research solutions, peer-reviewed journals, scientific conferences, eBooks and weekly news periodical Chemical & Engineering News. ACS journals are among the most cited, most trusted and most read within the scientific literature; however, ACS itself does not conduct chemical research. As a leader in scientific information solutions, its CAS division partners with global innovators to accelerate breakthroughs by curating, connecting and analyzing the world’s scientific knowledge. ACS’ main offices are in Washington, D.C., and Columbus, Ohio.
Here’s a link to and a citation for the paper,
Paintable Bioactive Extracellular Vesicle Ink for Wound Healing by Li Li, Zhiyu Wang, Kepeng Wang, Siyuan Fu, Dan Li, Mao Wang, Yi Cao, Houjuan Zhu, Ziyan Li, Lixing Weng, Zhiyang Li, Xianguang Ding, and Lianhui Wang. ACS Appl. Mater. Interfaces 2023, 15, 21, 25427–25436 DOI: https://doi.org/10.1021/acsami.3c03630 Publication Date:May 19, 2023 Copyright © 2023 American Chemical Society
This paper is behind a paywall.
