Researchers from China have developed a new type of yarn for flexible electronics. A March 28, 2018 news item on Nanowerk announces the work, (Note: A link has been removed),
When someone thinks about knitting, they usually don’t conjure up an image of sweaters and scarves made of yarn that can power watches and lights. But that’s just what one group is reporting in ACS Nano (“Waterproof and Tailorable Elastic Rechargeable Yarn Zinc Ion Batteries by a Cross-Linked Polyacrylamide Electrolyte”). They have developed a rechargeable yarn battery that is waterproof and flexible. It also can be cut into pieces and still work.
A March 28, 2018 2018 American Chemical Society (ACS) news release (also on EurekAlert), which originated the news item, expands on the theme (Note: Links have been removed),
Most people are familiar with smartwatches, but for wearable electronics to progress, scientists will need to overcome the challenge of creating a device that is deformable, durable, versatile and wearable while still holding and maintaining a charge. One dimensional fiber or yarn has shown promise, since it is tiny, flexible and lightweight. Previous studies have had some success combining one-dimensional fibers with flexible Zn-MnO2 batteries, but many of these lose charge capacity and are not rechargeable. So, Chunyi Zhi and colleagues wanted to develop a rechargeable yarn zinc-ion battery that would maintain its charge capacity, while being waterproof and flexible.
The group twisted carbon nanotube fibers into a yarn, then coated one piece of yarn with zinc to form an anode, and another with magnesium oxide to form a cathode. These two pieces were then twisted like a double helix and coated with a polyacrylamide electrolyte and encased in silicone. Upon testing, the yarn zinc-ion battery was stable, had a high charge capacity and was rechargeable and waterproof. In addition, the material could be knitted and stretched. It also could be cut into several pieces, each of which could power a watch. In a proof-of-concept demonstration, eight pieces of the cut yarn battery were woven into a long piece that could power a belt containing 100 light emitting diodes (known as LEDs) and an electroluminescent panel.
The authors acknowledge funding from the National Natural Science Foundation of China and the Research Grants Council of Hong Kong Joint Research Scheme, City University of Hong Kong and the Sichuan Provincial Department of Science & Technology.
Here’s an image the researchers have used to illustrate their work,
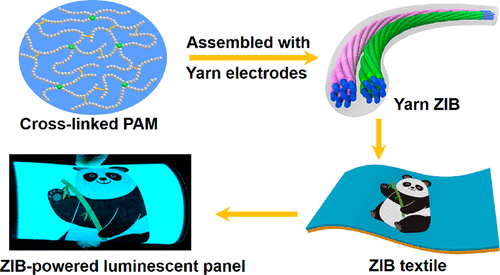
Courtesy: American Chemical Society
Here’s a link to and a citation for the paper,
Waterproof and Tailorable Elastic Rechargeable Yarn Zinc Ion Batteries by a Cross-Linked Polyacrylamide Electrolyte by Hongfei Li, Zhuoxin Liu, Guojin Liang, Yang Huang, Yan Huang, Minshen Zhu, Zengxia Pe, Qi Xue, Zijie Tang, Yukun Wang, Baohua Li, and Chunyi Zhi. ACS Nano, Article ASAP DOI: 10.1021/acsnano.7b09003 Publication Date (Web): March 28, 2018
Copyright © 2018 American Chemical Society
This paper is behind a paywall.

