
Parachute (sculpted felt lantern). Artist and artisan felter: Chantal Cardinal. Studio: FELT à la main with LOVE
Scientists from Kiel University (Christian-Albrechts-Universität zu Kiel; Germany) and the University of Trento (Italy) claim to have developed a new method for integrating carbon nanotubes (CNTs) into new materials in a technique they describe as similar to felting according to a November 21, 2017 news item on Nanowerk,
Extremely lightweight, electrically highly conductive, and more stable than steel: due to their unique properties, carbon nanotubes would be ideal for numerous applications, from ultra-lightweight batteries to high-performance plastics, right through to medical implants. However, to date it has been difficult for science and industry to transfer the extraordinary characteristics at the nanoscale into a functional industrial application. The carbon nanotubes either cannot be combined adequately with other materials, or if they can be combined, they then lose their beneficial properties.
Scientists from the Functional Nanomaterials working group at Kiel University (CAU) and the University of Trento have now developed an alternative method, with which the tiny tubes can be combined with other materials, so that they retain their characteristic properties. As such, they “felt” the thread-like tubes into a stable 3D network that is able to withstand extreme forces.
In contrast to the ‘felted’ image which opened this posting, here’s an image of the ‘felted’ carbon nanotubes,
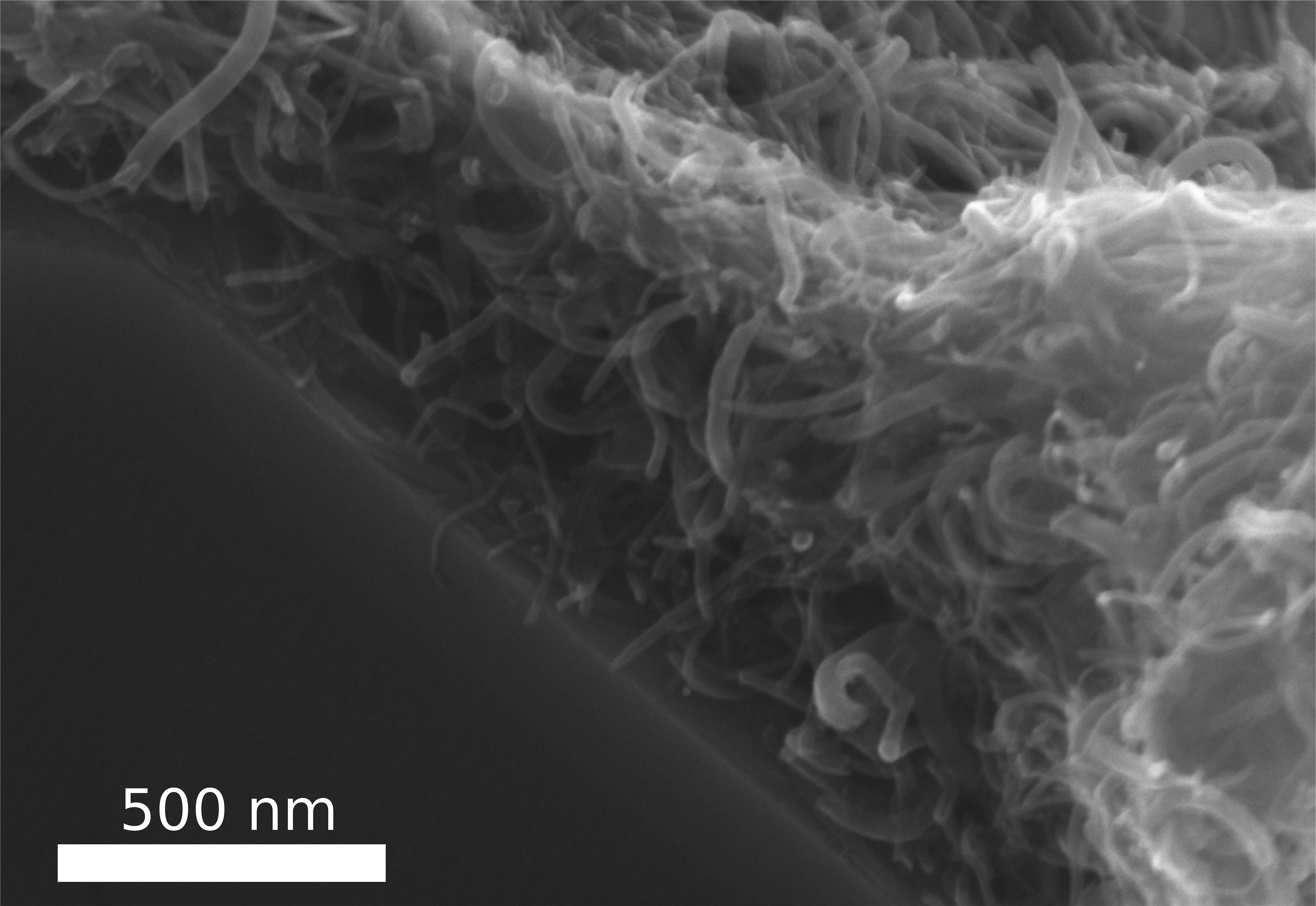
In this new process, the tiny, thread-like carbon nanotubes (CNTs) arrange themselves – almost like felting – to form a stable, tear-resistant layer. Photo/Copyright: Fabian Schütt Courtesy: Kiel University
A November 21, 2017 Kiel University press release (also on EurekAlert), which originated the news item, expands on the theme and adds another analogy,
Industry and science have been intensively researching the significantly less than one hundred nanometre wide carbon tubes (carbon nanotubes, CNTs), in order to make use of the extraordinary properties of rolled graphene. Yet much still remains just theory. “Although carbon nanotubes are flexible like fibre strands, they are also very sensitive to changes,” explained Professor Rainer Adelung, head of the Functional Nanomaterials working group at the CAU. “With previous attempts to chemically connect them with other materials, their molecular structure also changed. This, however, made their properties deteriorate – mostly drastically.”
In contrast, the approach of the research team from Kiel and Trento is based on a simple wet chemical infiltration process. The CNTs are mixed with water and dripped into an extremely porous ceramic material made of zinc oxide, which absorbs the liquid like a sponge. The dripped thread-like CNTs attach themselves to the ceramic scaffolding, and automatically form a stable layer together, similar to a felt. The ceramic scaffolding is coated with nanotubes, so to speak. This has fascinating effects, both for the scaffolding as well as for the coating of nanotubes.
On the one hand, the stability of the ceramic scaffold increases so massively that it can bear 100,000 times its own weight. “With the CNT coating, the ceramic material can hold around 7.5kg, and without it just 50g – as if we had fitted it with a close-fitting pullover made of carbon nanotubes, which provide mechanical support,” summarised first author Fabian Schütt. “The pressure on the material is absorbed by the tensile strength of the CNT felt. Compressive forces are transformed into tensile forces.”
The principle behind this is comparable with bamboo buildings [emphasis mine], such as those widespread in Asia. Here, bamboo stems are bound so tightly with a simple rope that the lightweight material can form extremely stable scaffolding, and even entire buildings. “We do the same at the nano-scale with the CNT threads, which wrap themselves around the ceramic material – only much, much smaller,” said Helge Krüger, co-author of the publication.
The materials scientists were able to demonstrate another major advantage of their process. In a second step, they dissolved the ceramic scaffolding by using a chemical etching process. All that remains is a fine 3D network of tubes, each of which consists of a layer of tiny CNT tubes. In this way, the researchers were able to greatly increase the felt surface, and thus create more opportunities for reactions. “We basically pack the surface of an entire beach volleyball field into a one centimetre cube,” explained Schütt. The huge hollow spaces inside the three-dimensional structure can then be filled with a polymer. As such, CNTs can be connected mechanically with plastics, without their molecular structure – and thus their properties – being modified. “We can specifically arrange the CNTs and manufacture an electrically conductive composite material. To do so only requires a fraction of the usual quantity of CNTs, in order to achieve the same conductivity,” said Schütt.
Applications for use range from battery and filter technology as a filling material for conductive plastics, implants for regenerative medicine, right through to sensors and electronic components at the nano-scale. The good electrical conductivity of the tear-resistant material could in future also be interesting for flexible electronics applications, in functional clothing or in the field of medical technology, for example. “Creating a plastic which, for example, stimulates bone or heart cells to grow is conceivable,” said Adelung. Due to its simplicity, the scientists agree that the process could also be transferred to network structures made of other nanomaterials – which will further expand the range of possible applications.
So, we have ‘felting’ and bamboo buildings. I can appreciate the temptation to use multiple analogies especially since I’ve given into it, on occasion. But, it’s never considered good style, not even when I do it.
Getting back to the work at hand, here’s a link to and a citation for the paper,
Hierarchical self-entangled carbon nanotube tube networks by Fabian Schütt, Stefano Signetti, Helge Krüger, Sarah Röder, Daria Smazna, Sören Kaps, Stanislav N. Gorb, Yogendra Kumar Mishra, Nicola M. Pugno, & Rainer Adelung. Nature Communications 8, Article number: 1215 (2017) doi:10.1038/s41467-017-01324-7 Published online: 31 October 2017
This is an open access paper.
One final comment, I notice that one of the authors is Nicola Pugno who was last mentioned here in an August 30, 2017 posting titled: Making spider silk stronger by feeding graphene and carbon nanotubes to spiders.
![Among the Picasso paintings in the Art Institute of Chicago collection, The Red Armchair is the most emblematic of his Ripolin usage and is the painting that was examined with APS X-rays at Argonne National Laboratory. To view a larger version of the image, click on it. Courtesy Art Institute of Chicago, Gift of Mr. and Mrs. Daniel Saidenberg (AIC 1957.72) © Estate of Pablo Picasso / Artists Rights Society (ARS), New York [downloaded from http://www.anl.gov/articles/high-energy-x-rays-shine-light-mystery-picasso-s-paints]](http://www.frogheart.ca/wp-content/uploads/2013/02/PicassoRedArmchair.jpg)