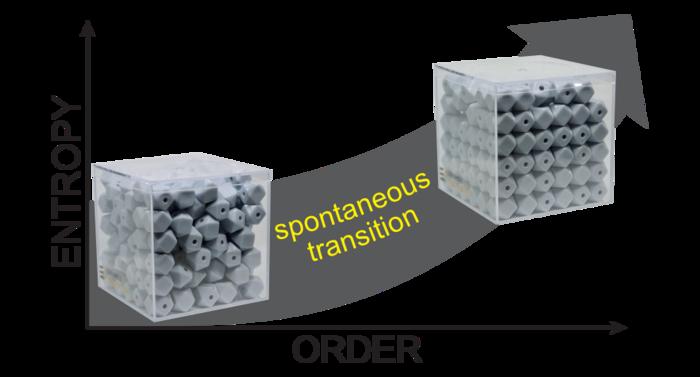
Using dice and buttons for understanding entropy? Apparently, filling the boxes in the image below with everyday objects helps students to better understand entropy and thermodynamics,
A September 6, 2023 news item on ScienceDaily describes a new approach to teaching entropy,
Though a cornerstone of thermodynamics, entropy remains one of the most vexing concepts to teach budding physicists in the classroom. As a result, many people oversimplify the concept as the amount of disorder in the universe, neglecting its underlying quantitative nature.
In The Physics Teacher, co-published by AIP [American Institute of Physics] Publishing and the American Association of Physics Teachers, researcher T. Ryan Rogers designed a hand-held model to demonstrate the concept of entropy for students. Using everyday materials, Rogers’ approach allows students to confront the topic with new intuition — one that takes specific aim at the confusion between entropy and disorder.
…
A September 6, 2023 AIP news release (also on EurekAlert), which originated the news item, provides more detail,
“It’s a huge conceptual roadblock,” Rogers said. “The good news is that we’ve found that it’s something you can correct relatively easily early on. The bad news is that this misunderstanding gets taught so early on.”
While many classes opt for the imperfect, qualitative shorthand of calling entropy “disorder,” it’s defined mathematically as the number of ways energy can be distributed in a system. Such a definition merely requires students to understand how particles store energy, formally known as “degrees of freedom.”
To tackle the problem, Rogers developed a model in which small objects such as dice and buttons are poured into a box, replicating a simple thermodynamic system. Some particles in the densely filled box are packed in place, meaning they have fewer degrees of freedom, leading to an overall low-entropy system.
As students shake the box, they introduce energy into the system, which loosens up locked-in particles. This increases the overall number of ways energy can be distributed within the box.
“You essentially zoom in on entropy so students can say, ‘Aha! There is where I saw the entropy increase,’” Rogers said.
As students shake further, the particles settle into a configuration that more evenly portions out the energy among them. The catch: at this point of high entropy, the particles fall into an orderly alignment.
“Even though it looks more orientationally ordered, there’s actually higher entropy,” Rogers said.
All the students who participated in the lesson were able to reason to the correct definition of entropy after the experiment.
Next, Rogers plans to extend the reach of the model by starting a conversation about entropy with other educators and creating a broader activity guide for ways to use the kits for kindergarten through college. He hopes his work inspires others to clarify the distinction in their classrooms, even if by DIY means.
“Grapes and Cheez-It crackers are very effective, as well,” Rogers said.
Here’s a link to and a citation for the paper,
Hands-on Model for Investigating Entropy and Disorder in the Classroom by T. Ryan Rogers. Phys. Teach. 61, 439–443 (2023) DOI: https://doi.org/10.1119/5.0089761 Published online September 1, 2023
This paper is open access.
I prefer this image of the teaching tool,